Abstract
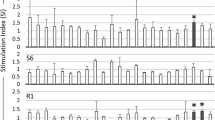
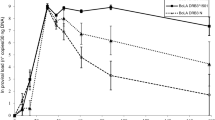
Major histocompatibility complex (MHC) is the best-characterized genetic region associated with resistance and susceptibility to a wide range of diseases. In cattle, the most important example of the relationship between the MHC and infectious diseases has been established by the resistance to Bovine leukemia virus (BLV) infection. The association of the bovine MHC class II BoLA-DRB3.2 alleles with BLV infection profiles was examined. BoLA-DRB3.2 allelic diversity was determined in 190 Iranian Holstein cattle using direct sequencing method. Association of the DRB3.2 alleles with BLV infection profiles was found as the odds ratio. Effects of the alleles on lymphocyte subsets were also evaluated by multivariate regression analysis and GLM procedures. The studied cattle were categorized into three groups: BLV seronegative, BLV seropositive with persistent lymphocytosis (PL), and BLV seropositive with lymphosarcoma (LS). The PL profile was significantly associated with the BoLA-DRB3.2*0101, *1101 and *4201 alleles, although the *3202 allele mediating resistance to PL was observed. Significant association was found between the BoLA-DRB3.2*1802, *3202, and *0901 alleles and susceptibility to LS, while the *0101 and *1101 alleles were associated with resistance to LS. BoLA-DRB3.2 alleles also showed a significant correlation with CD4, CD8, CD21 cells and CD4/CD8 ratio. Allelic differences influence the immune response to BLV infection and developing the disease profile. These differences also have important consequences for tumor resistance.
Similar content being viewed by others
References
Ballingall KT, Luyai A, Rowlands GJ, Sales J, Musoke AJ, Morzaria SP, McKeever DJ (2004) Bovine leukocyte antigen major histocompatibility complex class II DRB3* 2703 and DRB3* 1501 alleles are associated with variation in levels of protection against Theileria parva challenge following immunization with the sporozoite p67 antigen. Infect Immun 72:2738–2741
Baxter R, Craigmile S, Haley C, Douglas A, Williams J, Glass E (2009) BoLA-DR peptide binding pockets are fundamental for foot-and-mouth disease virus vaccine design in cattle. Vaccine 28:28–37
Burny A, Bruck C, Cleuter Y, Couez D, Deschamps J, Ghysdael J et al (1985) Bovine leukemia virus, a versatile agent with various pathogenic effects in various animal species. Cancer Res 45:4578–4582
Dietz AB, Cohen ND, Timms L, Kehrli ME Jr (1997) Bovine lymphocyte antigen class II alleles as risk factors for high somatic cell counts in milk of lactating dairy cows. J Dairy Sci 80:406–412
Esteban E, Poli M, Poiesz B, Ceriani CS, Gutierrez S, Dolcini G, Gagliardi R, Perez S, Lützelschwab C (2009) Bovine leukemia virus (BLV), proposed control and eradication programs by marker assisted breeding of genetically resistant cattle. Animal Genetics, 1st edn. Nova Science, New York, pp 107–130
Ewald S, Ye X, Avendano S, McLeod S, Lamont S, Dekkers J (2007) Associations of BF2 alleles with antibody titres and production traits in commercial pure line broiler chickens. Anim Genet 38:174–176
Ferrer JF (1980) Bovine lymphosarcoma. Adv Vet Sci Comp Med 24:1–67
Garcıa-Briones M, Russell G, Oliver R, Tami C, Taboga O, Carrillo E, Palma EL, Sobrino F, Glass E (2000) Association of bovine DRB3 alleles with immune response to FMDV peptides and protection against viral challenge. Vaccine 19:1167–1171
Gelhaus A, Schnittger L, Mehlitz D, Horstmann R, Meyer C (1995) Sequence and PCR-RFLP analysis of 14 novel BoLA-DRB3 alleles. Anim Genet 26:147–153
Juliarena M, Poli M, Sala L, Ceriani C, Gutierrez S, Dolcini G, Rodríguez E, Mariño B, Rodríguez-Dubra C, Esteban E (2008) Association of BLV infection profiles with alleles of the BoLA-DRB3. 2 gene. Anim Genet 39:432–438
Kabeya H, Ohashi K, Onuma M (2001) Host immune responses in the course of bovine leukemia virus infection. J Vet Med Sci 63:703–708
Korwin-Kossakowska A (2008) An association of BoLA alleles DRB3. 2* 16 and DRB3. 2* 23 with occurrence of mastitis caused by different bacterial species in two herds of dairy cows. Anim Sci Pap Rep 26:37–48
Lewin HA, Wu MC, Stewart JA, Nolan TJ (1988) Association between BoLA and subclinical bovine leukemia virus infection in a herd of Holstein-Friesian cows. Immunogenetics 27:338–344
Lewin HA, Russell GC, Glass EJ (1999) Comparative organization and function of the major histocompatibility complex of domesticated cattle. Immunol Rev 167:145–158
Maillard JC, Renard C, Chardon P, Bensaid A (1999) Characterization of 18 new BoLA-DRB3 alleles. Anim Genet 30:200–203
Mammerickx M, Lorenz RJ, Straub OC, Donnelly WJ, Flensburg JC, Gentile G, Markson LM, Ressang AA, Taylor SM (1978) Bovine hematology. IV. Comparative breed studies on the erythrocyte parameters of 16 European cattle breeds as determined in the Common Reference Laboratory. Zbl Vet Med B 25:484–498
Mirsky M, Olmstead C, Da Y, Lewin H (1998) Reduced bovine leukaemia virus proviral load in genetically resistant cattle. Anim Genet 29:245–252
Miyasaka T, Takeshima SN, Jimba M, Matsumoto Y, Kobayashi N, Matsuhashi T, Sentsui H, Aida Y (2013) Identification of bovine leukocyte antigen class II haplotypes associated with variations in bovine leukemia virus proviral load in Japanese Black cattle. Tissue Antigens 8:72–82
Murphy FA, Gibbs EPJ, Horzinek MC, Studdert MJ (1999) Veterinary virology. Academic Press, San Diego, California, USA
Otsuka M, Yakushijin Y, Hamada M, Hato T, Yasukawa M, Fujita S (2004) Role of CD21 antigen in diffuse large B-cell lymphoma and its clinical significance. Br J Haemato 127:416–424
Panei C, Suzuki K, Echeverria M, Serena M, Metz G, Gonzalez E (2009) Association of BoLA-DRB3. 2 alleles with resistance and susceptibility to persistent lymphocytosis in BLV infected cattle in Argentina. Int J Dairy Sci 4:123–128
Park YH, Park JY, Kim SH, Ahn JS, Davies CJ (2004) Characterization of lymphocyte subpopulations and major histocompatibility complex haplotypes of mastitis-resistant and susceptible cows. J Vet Sci 5:29–39
Pashmi M, Qanbari S, Ghorashi S, Sharifi A, Simianer H (2009) Analysis of relationship between bovine lymphocyte antigen DRB3. 2 alleles, somatic cell count and milk traits in Iranian Holstein population. J Anim Breed Genet 126:296–303
Rupp R, Hernandez A, Mallard B (2007) Association of bovine leukocyte antigen (BoLA) DRB3. 2 with immune response, mastitis, and production and type traits in Canadian Holsteins. J Dairy Sci 90:1029–1038
Sharif S, Mallard B, Wilkie B, Sargeant J, Scott H, Dekkers J, Leslie K (1998) Associations of the bovine major histocompatibility complex DRB3 (BoLA-DRB3) alleles with occurrence of disease and milk somatic cell score in Canadian dairy cattle. Anim Genet 29:185–193
Stear M, Dimmock C, Newman M, Nicholas F (1988) BoLA antigens are associated with increased frequency of persistent lymphocytosis in bovine leukaemia virus infected cattle and with increased incidence of antibodies to bovine leukaemia virus. Anim Genet 19:151–158
Stear MJ, Nikbakht G, Matthews L, Jonsson NN (2012) Breeding for disease resistance in livestock and fish. Anim Sci Rev 7:1–10
Tiwari JL, Terasaki PI (1985) HLA and disease associations. Springer, New York
Van Eijk M, Stewart-haynes J, Lewin H (1992) Extensive polymorphism of the BOLA-DRB3 gene distinguished by PCR-RFLP. Anim Genet 23:483–496
Wang H, Norris KM, Mansky LM (2002) Analysis of bovine leukemia virus gag membrane targeting and late domain function. J Virol 76:8485–8493
Xu A, Van Eijk M, Park C, Lewin HA (1993) Polymorphism in BoLA-DRB3 exon 2 correlates with resistance to persistent lymphocytosis caused by bovine leukemia virus. J Immunol 151:6977–6985
Yeh F, Yang R, Boyle TJ, Mao J (1997) POPGENE Software: microsoft windows-based freeware for population genetic analysis (version 1.32(. Center for International Forestry Research, University of Alberta, Edmonton, Canada
Zanotti M, Poli G, Ponti W, Polli M, Rocchi M, Bolzani E, Longeri M, Russo S, Lewin H, Van Eijk M (1996) Association of BoLA class II haplotypes with subclinical progression of bovine leukaemia virus infection in Holstein-Friesian cattle. Anim Genet 27:337–341
Zavala-Ruiz Z, Strug I, Walker BD, Norris PJ, Stern LJ (2004) A hairpin turn in a class II MHC-bound peptide orients residues outside the binding groove for T cell recognition. Proc Natl Acad Scis USA 101:13279–13284
Acknowledgments
This work was supported by the Iran National Science foundation, Grant No. 910027.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nikbakht Brujeni, G., Ghorbanpour, R. & Esmailnejad, A. Association of BoLA-DRB3.2 Alleles with BLV Infection Profiles (Persistent Lymphocytosis/Lymphosarcoma) and Lymphocyte Subsets in Iranian Holstein Cattle. Biochem Genet 54, 194–207 (2016). https://doi.org/10.1007/s10528-016-9712-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-016-9712-6