Abstract
Generalist predators are often used in biological control programs, although they can be detrimental for pest control through interference with other natural enemies. Here, we assess the effects of generalist natural enemies on the control of two major pest species in sweet pepper: the green peach aphid Myzus persicae (Sulzer) and the western flower thrips Frankliniella occidentalis (Pergande). In greenhouses, two commonly used specialist natural enemies of aphids, the parasitoid Aphidius colemani Viereck and the predatory midge Aphidoletes aphidimyza (Rondani), were released together with either Neoseiulus cucumeris Oudemans, a predator of thrips and a hyperpredator of A. aphidimyza, or Orius majusculus (Reuter), a predator of thrips and aphids and intraguild predator of both specialist natural enemies. The combined use of O. majusculus, predatory midges and parasitoids clearly enhanced the suppression of aphids and consequently decreased the number of honeydew-contaminated fruits. Although intraguild predation by O. majusculus on predatory midges and parasitoids will have affected control of aphids negatively, this was apparently offset by the consumption of aphids by O. majusculus. In contrast, the hyperpredator N. cucumeris does not prey upon aphids, but seemed to release aphids from control by consuming eggs of the midge. Both N. cucumeris and O. majusculus did not affect rates of aphid parasitism by A. colemani. Thrips were also controlled effectively by O. majusculus. A laboratory experiment showed that adult predatory bugs feed on thrips as well as aphids and have no clear preference. Thus, the presence of thrips probably promoted the establishment of the predatory bugs and thereby the control of aphids. Our study shows that intraguild predation, which is potentially negative for biological control, may be more than compensated by positive effects of generalist predators, such as the control of multiple pests, and the establishment of natural enemies prior to pest invasions. Future work on biological control should focus on the impact of species interactions in communities of herbivorous arthropods and their enemies.
Similar content being viewed by others
Introduction
Generalist predators are increasingly used to control multiple pests in biological control programs (Chang and Kareiva 1999; Symondson et al. 2002; Sabelis et al. 2008; Messelink et al. 2010). Generalist predatory mites and predatory bugs are among the most successful control agents against common greenhouse pests such as thrips, whiteflies, spider mites and aphids (Gerson and Weintraub 2007; Sabelis et al. 2008, Cock et al. 2010). An important reason for this success is the ability of these predators to colonize crops when pests are absent or present at low densities because they can feed on alternative food sources. This can result in high predator densities relative to those of the invading prey, thereby preventing a pest outbreak. Another reason is that generalist predators can be very effective in suppressing multiple species of plant pests. Several studies have shown that predator-mediated interactions between pest species (apparent competition; Holt 1977) can enhance pest control within a time scale relevant to pest control programs (e.g., Karban et al. 1994; Hanna et al. 1997; Harmon and Andow 2004; Messelink et al. 2008, 2010).
However, many generalist predators do not only feed on pests or plant-provided food, but also on other natural enemies, which can be detrimental for biological control (Rosenheim et al. 1995; Rosenheim 1998; Snyder and Ives 2001; Symondson et al. 2002; Janssen et al. 2007). This feeding on other natural enemies can be classified as intraguild predation when the enemies share a prey and thus compete for it (Polis et al. 1989; Holt and Polis 1997; Rosenheim et al. 1995). Predators can also attack other predators with which they do not share a prey. The consumption of predators by other predators has been referred to as “secondary predation” (Rosenheim et al. 1995), or “hyperpredation” (Müller and Brodeur 2002; Messelink et al. 2011), or “higher-order predation” (Rosenheim 1998; Symondson et al. 2002). This last definition includes both hyperpredation and intraguild predation. Here, we use hyperpredation for predators eating other predators without sharing a prey because it has a clear parallel to the term “hyperparasitism”.
Basic theory about species interactions helps to understand the dynamics of pest-predator interactions, but is often limited to relatively simple systems with only two predators and one prey species (Holt and Polis 1997). Recent studies have extended this theory by including food web complexity, such as alternative prey effects (Holt and Huxel 2007) or spatial heterogeneity (Heithaus 2001). However, real-life predator–prey systems are often embedded in more complex communities with several interacting species, and there is no theory for such systems. Many ecologists have recognized this complexity and suggested more empirical studies that test multiple species interactions in realistic natural enemy communities (Rosenheim et al. 1995; Cardinale et al. 2003; Letourneau et al. 2009). Such studies are of major importance for developing biological control strategies, for example in greenhouse crops where artificial communities are created by releases of several species of natural enemies (van Lenteren 2000; Enkegaard and Brødsgaard 2006).
Our main goal is to determine the relative importance of interactions with negative (i.e., hyperpredation and intraguild predation) and positive (i.e., apparent competition) effects on pest control, in a food web of plant pests and their natural enemies. In a multi-species experiment, we assessed the effects of specialist and generalist enemies on the suppression of two major co-occurring pest species in sweet pepper: the green peach aphid Myzus persicae (Sulzer) and western flower thrips Frankliniella occidentalis (Pergande). Current biological control programs often fail in suppressing aphids (Bloemhard and Ramakers 2008) and one reason for this might be that generalist thrips predators interact with specialist aphid natural enemies. Biological control of thrips in sweet pepper is usually achieved through releases of generalist predatory bugs of the genus Orius in combination with generalist phytoseiid mites (Shipp and Ramakers 2004). Aphids are usually controlled through the release of a combination of specialised parasitoids (mainly Aphididae) and the specialist predatory midge Aphidoletes aphidimyza (Rondani) (Blümel 2004). The midges are mainly released for controlling high densities of aphids because specialist parasitoids cannot establish control fast enough. Yet, parasitoids are generally preferred for aphid control at low densities because it is cheaper. Recently, we demonstrated that generalist predatory mites used for thrips control can seriously disrupt biological control of aphids by preying on the eggs of predatory midges (Messelink et al. 2011). Because these predatory mites do not kill aphids, and thus do not share prey with the predatory midges, they can be classified as hyperpredators. In contrast, Orius bugs prey on eggs and larvae of A. aphidimyza (Christensen et al. 2002; Hosseini et al. 2010), but also on aphids (Alvarado et al. 1997) and therefore act as intraguild predators. Moreover, they are intraguild predators of parasitoids by preying on parasitized aphids (Snyder and Ives 2003). We compared the effects of these two types of interaction, hyperpredation versus intraguild predation, on the control of thrips and aphids in a setting with the hyperpredator Neoseiulus cucumeris Oudemans or the intraguild predator Orius majusculus (Reuter) (Fig. 1) together with A. aphidimyza and the parasitoid Aphidius colemani Viereck. In both food webs, intraguild predation of parasitized aphids by the predatory midge A. aphidimyza also occurs (Brodeur and Rosenheim 2000, Fig. 1). We hypothesized that disruption of aphid control will be stronger with hyperpredators than with intraguild predators, because the hyperpredators only feed on the other natural enemies, whereas the intraguild predators feed on these enemies as well as on the aphids. Moreover, the presence of thrips may contribute to the control of aphids by increasing population densities of the intraguild predators. However, this only applies when the intraguild predators do not have a strong preference for either thrips or aphids. To test this, we observed predation and oviposition rates of O. majusculus on both prey when present separately or simultaneously on leaf discs in the laboratory. These results may help to understand which underlying mechanisms are responsible for effects of different natural enemy assemblages on pest control.
Two strategies for biological control of thrips and aphids in sweet pepper. Arrows indicate consumption of the species at the tip of the arrow by the species at the base of the arrow. Strategy A involves hyperpredation of aphid predatory midges by predatory mites, whereas strategy B involves intraguild predation of aphid predatory midges and parasitized aphids by predatory bugs
Materials and methods
Plants, insects and mites
Sweet pepper plants (Capsicum annuum L. cv. Spider) were grown by a commercial plant propagator in rock wool blocks in a greenhouse, where they were treated twice with a 0.05 % solution of abamectine (Vertimec®, Syngenta) to keep them free of pests. Green peach aphids, M. persicae, of the red phenotype were reared on sweet pepper plants cv. Spider in a greenhouse compartment. Western flower thrips, F. occidentalis, were reared on flowering chrysanthemum plants (Dendranthema grandiflora Tzvelev, cv. Miramar) in a separate greenhouse compartment. Predatory mites N. cucumeris, predatory midges A. aphidimyza and the aphid parasitoids A. colemani were obtained from Koppert Biological Systems (Berkel en Rodenrijs, The Netherlands). The predatory bugs O. majusculus were obtained from Biobest NV (Westerlo, Belgium). For the prey preference and oviposition experiment, we maintained a laboratory culture of this predatory bug with eggs of the flour moth Ephestia kuehniella Zeller as food and bean pods (Phaseolus vulgaris L.) as oviposition sites, following methods described by van den Meiracker and Ramakers (1991). The culture was kept in a climate room at 25 °C, 70 % RV and a photoperiod of 16L:8D. In order to produce second-instar thrips larvae for the laboratory experiment, thrips females were collected from the culture on chrysanthemum and offered fresh bean pods as oviposition substrate, in glass jars, which were closed with lids equipped with a mesh (size 80 μm) to allow ventilation. After 2–3 days the adult thrips were removed and the larvae that emerged from the eggs were grown on the same pods until they reached the second instar. Thrips larvae were reared in a separate climate chamber, under the same conditions as O. majusculus.
Greenhouse experiments
Greenhouse experiments were conducted in a row of six bordering compartments, 24 m2 each, at the institute of Greenhouse Horticulture (Wageningen UR). The windows of these compartments were provided with insect gauze (mesh size 0.40 × 0.45 mm) to exclude contamination with organisms from outside. Sweet pepper plants cv Spider were planted in March 2009 in each compartment in four rows, with nine plants per row. Plants were grown according to standard cultivation methods on rock wool slabs with drip irrigation for supplying water and nutrients.
The following natural enemy assemblages were compared: (1) control treatment with releases of specialist aphid parasitoids and predators (A. colemani and A. aphidimyza), (2) the hyperpredator A. cucumeris together with A. colemani and A. aphidimyza (strategy A, Fig. 1) and (3) the intraguild predator O. majusculus together with A. colemani and A. aphidimyza (strategy B, Fig. 1). Each treatment was applied in two compartments and each compartment was divided in two fields of 18 plants each. Because the fields were spatially separated by a path between the plant rows, we considered each field as a separate experimental unit. However, some exchange of flying stages of the released species between two fields in one greenhouse compartment might have occurred. The predators N. cucumeris and O. majusculus were released four weeks prior to the pest species on flowering sweet pepper plants of ca. 0.8 m height. The predators can survive and reproduce on such plants because of the presence of sweet pepper pollen as food. This release schedule mimics the situation in commercial greenhouses (Shipp and Ramakers 2004). Adult O. majusculus were released in the middle of each field at densities of 100 adults (60 % female) per field (=5.5 adults plant−1), which was repeated after three weeks to ensure establishment (Table 1). Predatory mites (N. cucumeris) were released once at densities of ca. 100 mites (mixed age) per plant (1,800 field−1) by sprinkling the commercial product (bran with the storage mite Tyrophagus putrescentiae (Schrank) and predatory mites) on top of the plants. Release densities were determined by counting the number of predatory mites per gram of product in the laboratory under a binocular microscope (40×), after washing and sieving the material over a 400 and 63 μm sieve. Starting four weeks after the first releases of N. cucumeris and O. majusculus (Table 1), individual aphids were transferred from the culture on sweet pepper to the upper leaves of each plant with a fine paintbrush at densities of 2, 4 or 8 per plant (Table 1). Thrips were introduced at the same time, by collecting adult females with an aspirator from the culture on chrysanthemum, and releasing them at a rate of six per three plants (Table 1). The specialist natural enemies of aphids, A. aphidimyza and A. colemani, were released starting three weeks after the first pest introductions (Table 1). Release densities were higher in the last week because of a strong increase of aphid densities after a few hot days. The exact release densities of pests and natural enemies per field are presented in Table 1. Predatory midges and parasitoids were released as pupae and mummies respectively by putting them in a Petri dish with vermiculite (carrier material of the commercial product), which was placed on the ground in the shade, in the middle of each row. Densities of pests and predators were assessed weekly for a period of seven weeks, starting four weeks after the first pest introductions (Table 1). The numbers of aphids, O. majusculus, A. aphidimyza and parasitized aphids were counted per field on both sides of ten randomly chosen leaves in the upper plant layer and ten leaves in a layer that was about 0.5 m below the top of the plant. Parasitism was quantified by counting the number of mummies per leaf. These counts were cumulative, because mummies from which the parasitoid had already emerged were not separated from intact mummies. Thrips and predatory mites were more equally distributed on the plants than aphids, and their densities were assessed on eight randomly chosen leaves per field, which were assessed in the laboratory under a binocular microscope (40×).
Sweet pepper fruits were harvested as soon as they became red. The total production of peppers and the number of peppers severely contaminated by aphid honeydew was recorded per compartment during the entire experiment. Temperature and relative humidity in each greenhouse compartment were registered every 5 min throughout the experiment with a climate recorder. Conditions were nearly equal in all compartments, with average temperatures of 21.2 (±0.04 SE)°C and average relative humidities of 71 (±0.5 SE) %. Differences in population dynamics of pests and natural enemies among the treatments were analysed using generalized linear mixed effects models with time and compartment as random factors to correct for repeated measures and pseudoreplication within compartments. Poisson error distributions were applied for the average numbers of aphids, thrips, mummies and gall midges per leaf per field and a binomial error distribution was used for the average fractions of aphid parasitism per leaf per field. Effects of treatments on fruit yield and honeydew contamination were analysed with generalized linear mixed effects models with compartment as random factor to correct for pseudoreplication. A Poisson error distribution was applied for the total number of fruits per field and a binomial distribution for the fractions of contaminated fruit per field. Differences among treatments were tested at the 5 % level using Fisher’s LSD (Least Significant Difference) method.
Prey preference and oviposition rates of O. majusculus
A laboratory experiment was conducted to determine if O. majusculus feeds on thrips as well as aphids when presented together and to assess whether this predator has a strong preference for one of the two prey. This was done because a strong preference could affect pest control in the short term. Simultaneously, we assessed oviposition rates on diets of thrips, aphids and the mixture of the two pests to assess whether the predator can reproduce on both prey species. The experiment was conducted in a climate room under 16 h of artificial illumination per day, at 22 °C and 70 % RH. Predation and oviposition rates were measured with 1-week-old mated females (pre-oviposition period is 4–5 days at 26 °C, Tommasini et al. 2004), which were starved for one day on bean pods to ensure they were motivated to feed. We used plastic boxes (5 cm high, diameter 6 cm) with a sweet pepper leaf disc (diameter 6 cm) that was embedded upside-down in water agar (1 % agar), making the abaxial side of the discs available to the prey species and predators. Either 80 second instar thrips larvae, 80 third instar aphid nymphs or a mixture of 80 thrips larvae and 80 aphid nymphs were added to the discs, so ample prey was present in all treatments. Subsequently, one starved female O. majusculus was added to each box. The boxes were placed upside down on a tray covered with gauze in order to have the abaxial side of the discs facing downwards as on plants. Ventilation was possible through a hole in the lid covered with insect gauze (mesh size 80 μm). The bugs were transported to a new box with the same prey densities after 24, 48 and 72 h. Predation and oviposition rates were measured after the predators had been transferred. Eggs were mainly deposited in the leaf veins and could easily be counted under a binocular microscope (40×). For analysis of oviposition rates, data from the first and second day were omitted to reduce the influence of pre-experimental conditions. Each treatment was replicated 11 times. Average daily predation and oviposition rates were log-transformed, analysed with standard ANOVA and tested for differences among treatments at the 5 % level using Fisher’s LSD (Least Significant Difference) method. All statistical analyses were done with GenStat Release 13.2 (Payne et al. 2010).
Results
Greenhouse experiment
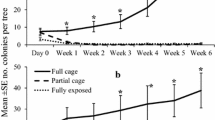
Aphids were effectively controlled in the treatment with predatory bugs + parasitoids + midges, significantly better than in the treatments with predatory mites + parasitoids + midges or parasitoids + midges (F 2,36 = 5.33, p = 0.009, Fig. 2a). Aphid densities increased rapidly to high numbers in the latter two treatments. The aphid densities in the treatment with predatory mites, parasitoids and midges were higher than those in the treatment with parasitoids and midges only, but this difference was not significant (Fig. 2a). Overall densities of thrips differed significantly among treatments (F 2,36 = 13.39, p < 0.001) and the best control was achieved in the treatment with predatory bugs plus the specialised aphid enemies (Fig. 2b).
Population dynamics of a the green peach aphid M. persicae and b western flower thrips F. occidentalis in a sweet pepper crop in the presence of three assemblages of natural enemies. All three treatments received parasitoids (A. colemani) plus predatory midges (A. aphidimyza). The generalist predatory mite N. cucumeris (treatment predatory mites + parasitoids + midges) or the generalist predatory bug O. majusculus (predatory bugs + parasitoids + midges) were furthermore released in two treatments prior to the aphid enemies (see Table 1 for release rates and times). Shown are average (±SE) densities per leaf. Different letters indicate significant differences among treatments through time (Fisher’s LSD test, p < 0.05)
Eventually, all aphids were parasitized by A. colemani in all treatments (Fig. 3a, b). Numbers of mummies in the treatment with predatory bugs were significantly lower than in the other treatments (F 2,36 = 3.62, p = 0.037, Fig. 3a), but the percentages of parasitism did not differ among treatments (F 2,36 = 0.06, p = 0.943, Fig. 3b). Densities of midges were significantly lower in the treatment with predatory bugs than in the other two treatments (F 2,33 = 5.61, p = 0.008, Fig. 3c). Predatory mite densities suddenly dropped between six and seven weeks after the first pest introductions, whereas densities of predatory bugs continued to increase (Fig. 3d). The better aphid control in the treatments with predatory bugs resulted in a significantly lower percentage of fruits contaminated with honeydew (F 2,3 = 32.58, p = 0.004, Fig. 4). Fruit yield was not significantly different among treatments (F 2,3 = 4.68, p = 0.120). Slight silver damage on the fruits, caused by thrips, was found occasionally and therefore not quantified.
Population dynamics of a, b the parasitoid A. colemani, c the predatory midge A. aphidimyza and d the predatory mite N. cucumeris and the predatory bug O. majusculus in a sweet pepper crop infested by the green peach aphid M. persicae and western flower thrips F. occidentalis. See legend to Fig. 2 for further explanation. Shown are average (±SE) percentages of parasitized aphids and average (±SE) densities of mummies, midge larvae and predators per leaf. Different letters indicate significant differences among treatments through time (Fisher’s LSD test, p < 0.05)
Total number (±SE) of clean and honeydew-contaminated pepper fruits from plants infested with the green peach aphid M. persicae and western flower thrips F. occidentalis in the presence of three assemblages of natural enemies. Fruit production was measured during 18 weeks. See legend to Fig. 2 for further explanation. Different letters within bars indicate significant differences in contamination with aphid honeydew among treatments (Fisher’s LSD test, p < 0.05)
Prey preference and oviposition rates of O. majusculus
All female O. majusculus consumed aphids as well as thrips when offered together, showing that they do not exclusively prefer either of the two prey (Fig. 5). The consumption of thrips larvae was significantly lower (43 %) in the presence of aphids (F 1,19 = 13.39, p = 0.002), whereas the consumption of aphids was not significantly changed by the presence of thrips (F 1,20 = 0.11, p = 0.743). Oviposition rates after 72 h did not differ significantly among the three diets (Fig. 6, F 2,30 = 1.26; p = 0.298).
Number of prey consumed by one-week-old adult females of O. majusculus per day when offered second instar thrips larvae and third instar aphid nymphs either separately or in combination (mixed diet). Shown are average numbers of prey consumed (±SE) per female per day (measured over three days). Different letters above bars indicate significant differences in consumption of thrips or aphids between the mixed pest treatment and the single pest treatment (Fisher’s LSD test, p < 0.05)
Discussion
We aimed to assess the impact of generalist predators involved in intraguild predation or hyperpredation on specialised natural enemies, herbivore densities and the yield in a sweet pepper crop. The hyperpredator N. cucumeris and intraguild predator O. majusculus were both expected to release aphids from control because both predators prey on the specialised natural enemies of the aphids. However, releasing O. majusculus together with predatory midges and parasitoids clearly improved aphid control. Thus, intraguild predation by O. majusculus on predatory midges and parasitoids did not release the aphids from control. Apparently, the effects of intraguild predation were outweighed by O. majusculus preying on aphids. As expected, the hyperpredator N. cucumeris did not affect aphid densities significantly. This corresponds with an earlier study, where N. cucumeris also did not significantly disrupt aphid control (Messelink et al. 2011). However, hyperpredation by the predatory mite Amblyseius swirskii (Athias-Henriot) on predatory midges clearly disrupted the biological control of aphids (Messelink et al. 2011). Yet, caution should be exercised, because the effects of hyperpredation may depend on the densities of the predatory mites (Messelink et al. 2011). Not only aphids, but also thrips were strongly suppressed by O. majusculus. Both pests were ultimately controlled in all treatments, but the treatments with predatory bugs had the lowest number of honeydew-contaminated fruits. It is not clear why thrips densities ultimately also went down in the treatment without thrips predators. The high aphid densities in this treatment possibly reduced plant quality and consequently thrips growth rates.
Our results do not provide evidence for strong negative or positive effects of the generalist predators on parasitoids. Possibly, such effects were not detected because of the repeated releases of adult parasitoids, which are invulnerable to predation. However, females of A. colemani live relatively short (ca. ten days) and most eggs are laid within the first three days after emerging from mummies (van Steenis 1993). Hence, we assume that the observed parasitism in the five weeks after the last parasitoid release was caused by the offspring of the released parasitoids, and these parasitoids had been exposed to intraguild predation. The absolute numbers of parasitized aphids were much lower in treatments with predatory bugs than in the other treatments, likely because the number of aphids available for parasitism was reduced by aphid consumption by the predatory bugs. However, the predatory bugs probably also consumed parasitized aphids. Because equal numbers of parasitoids were released in all treatments, the parasitoid:aphid ratio was higher in the treatments with predatory bugs because of the lower number of aphids. Thus, higher rates of parasitism were expected in the treatment with predatory bugs. This was not observed, perhaps as a result of intraguild predation of parasitized aphids by the predatory bugs. However, parasitoids may also have been less effective at these lower aphid densities because they had to spend more time on host searching.
One explanation for the excellent aphid control with O. majusculus is that predation on thrips and midges might have increased the densities of O. majusculus, which consequently increased predation on aphids. This so-called predator-mediated apparent competition between prey species can enhance pest control (Karban et al. 1994; Messelink et al. 2008; Yoo and O’Neil 2009). In addition to these prey, sweet pepper pollen probably also contributed to the establishment of the predatory bugs.
Besides the positive effects of thrips on the predators, we cannot exclude the possibility that the presence of thrips released aphids from control by predatory bugs in the short term (Desneux and O’Neil 2008), because we did not collect data during the first four weeks. Such an effect might even be stronger when the predatory bugs prefer thrips to aphids as prey (Desneux and O’Neil 2008). However, our laboratory experiment showed that adult predatory bugs did not exclusively prefer either of the two prey species, and reproduced on both prey species. Thus, the presence of thrips probably contributed to the control of aphids because it resulted in higher predatory bugs.
The opposite effect, the presence of aphids resulting in a release of thrips from control, might also have occurred in the short-term, especially because the presence of aphids reduced predation on thrips by the predatory bugs. This might have occurred in the first four weeks, when no data were collected. However, the low thrips densities after four weeks and the absence of significant crop damage by thrips suggests that, if present at all, such an effect was not strong.
Increased densities of O. majusculus through predation on thrips and aphids might have increased predation on parasitized aphids and midge eggs and larvae. Indeed, midge densities were lowest in the treatment with predatory bugs, possibly caused by predation of midges by predatory bugs and by competition between bugs and midges for aphids. Thus the decreased midge densities might have released aphids from control, but this effect was apparently outweighed by the predatory bugs consuming aphids.
Equilibrium theory on intraguild predation predicts that disruption of biological control only occurs when the intraguild prey is the better competitor for the shared pest than the intraguild predator (Holt and Polis 1997; Janssen et al. 2006). Although these predictions may not directly apply to dynamics at a shorter time scale (Briggs and Borer 2005), it is possible that our intraguild predator (O. majusculus) was a better competitor for aphids than the parasitoids and midges. In that case, theory predicts that the intraguild prey should be outcompeted by the intraguild predator, and indeed, the midges tended to disappear in the treatment with predatory bugs (Fig. 3b). Intraguild predation by predatory bugs on parasitoids and midges did not affect aphid control negatively. This corresponds with previous studies showing that intraguild predators may reduce densities of intraguild prey, but in general do not disrupt control of the shared prey (Janssen et al. 2006, 2007; Vance-Chalcraft et al. 2007).
Several studies with generalist predators found that predation rates increased in the presence of multiple prey species (Lucas et al. 2004; Madsen et al. 2004; Koss et al. 2004). Our laboratory experiment possibly indicates such effects for O. majusculus. Although predation rates on thrips decreased in the presence of aphids, the opposite was not the case. Thus, the total number of prey killed increased in the mixed diet relative to aphids as only prey. This effect was not caused by differences in prey density because ample prey was offered in all treatments.
So far, the biological control of aphids in greenhouses is mainly based on releases of specialised natural enemies (Ramakers 1989; Blümel 2004), perhaps based on criteria for selecting natural enemies that were advocated in the past (van Lenteren and Woets 1988). However, our study suggests that generalist predatory bugs, although potentially risky as intraguild predators, can play a major role in controlling aphids. They are able to respond rapidly to aphid infestations because of their continuous presence in a crop. One could argue that sufficient densities of these predators would even control aphids. However, inoculative releases of predatory bugs might not always be sufficient for suppressing high aphids densities because the generation time of predatory bugs is too long for a timely numerical response. In such cases, it might be better to additionally release enemies with a strong numerical response, such as parasitoids. Specialised aphid predators that can “clean up” dense aphid colonies, such as predatory midges, may also be necessary.
The hyperpredators mediate an indirect interaction between the alternative prey and the specialist predator (Fig. 1). This interaction can be classified as apparent competition, because the prey and specialist interact through a shared hyperpredator population (Holt 1977), however, they occupy different trophic levels. Theory on apparent competition predicts that the presence of one prey lowers the equilibrium densities of the second prey. For hyperpredation, this would mean lower equilibrium densities of the specialist predator, which could consequently release the prey of the specialist from control. Thus, it is expected that hyperpredators will decrease the densities of specialist predators that are vulnerable for hyperpredation, and consequently increase the densities of the prey of these specialists. The reason that we did not find a significant reduction of midge densities by the hyperpredator N. cucumeris may be that the high numbers of aphids contaminated the leaves with sticky honeydew, which may have reduced predatory mite activity (Nomikou et al. 2003). Preliminary results indeed showed that the presence of sticky honeydew hinders predatory mite movement and strongly reduced predation rates on thrips (Messelink personnel observation).
In conclusion, our study shows that potential negative effects of intraguild predation on biological control may be compensated by positive effects, such as the control of multiple pests by generalist (intraguild) predators, and the establishment of these predators prior to pest invasions. Thus, research on biological control should assess the impact of generalist predators in relevant pest-natural enemy communities.
References
Alvarado P, Balta O, Alomar O (1997) Efficiency of four heteroptera as predators of Aphis gossypii and Macrosiphum euphorbiae (Hom.: Aphididae). Entomophaga 42:215–226
Bloemhard CMJ, Ramakers PMJ (2008) Strategies for aphid control in organically grown sweet pepper in the Netherlands. IOBC/WPRS 32:25–28
Blümel S (2004) Biological control of aphids on vegetable crops. In: Heinz KM, van Driesche RG, Parrella MP (eds) Biocontrol in protected culture. Ball Publishing, Batavia, USA, pp 297–312
Briggs CJ, Borer ET (2005) Why short-term experiments may not allow long-term predictions about intraguild predation. Ecol Appl 15:1111–1117
Brodeur J, Rosenheim JA (2000) Intraguild interactions in aphid parasitoids. Entomol Exp Appl 97:93–108
Cardinale BJ, Harvey CT, Gross K, Ives AR (2003) Biodiversity and biocontrol: emergent impacts of a multi-enemy assemblage on pest suppression and crop yield in an agroecosystem. Ecol Lett 6:857–865
Chang GC, Kareiva P (1999) The case for indigenous generalists in biological control. In: Hawkins BA, Cornell HV (eds) Theoretical approaches to biological control. Cambridge University Press, Cambridge, UK, pp 103–115
Christensen RK, Enkegaard A, Brødsgaard HF (2002) Intraspecific interactions among the predators Orius majusculus and Aphidoletes aphidimyza. IOBC/WPRS Bull 25:57–60
Cock MJW, van Lenteren JC, Brodeur J, Barratt BIP, Bigler F, Bolckmans K, Consoli FL, Haas F, Mason PG, Parra JRP (2010) Do new access and benefit sharing procedures under the convention on biological diversity threaten the future of biological control? BioControl 55:199–218
Desneux N, O’Neil RJ (2008) Potential of an alternative prey to disrupt predation of the generalist predator, Orius insidiosus, on the pest aphid, Aphis glycines, via short-term indirect interactions. Bull Entomol Res 98:631–639
Enkegaard A, Brødsgaard HF (2006) Biocontrol in protected crops: is lack of biodiversity a limiting factor? In: Eilenberg J, Hokkanen HMT (eds) Ecological and societal approach to biological control. Springer, Dordrecht, The Netherlands, pp 91–122
Gerson U, Weintraub PG (2007) Mites for the control of pests in protected cultivation. Pest Manag Sci 63:658–676
Hanna R, Wilson LT, Zalom FG, Flaherty DL (1997) Effects of predation and competition on the population dynamics of Tetranychus pacificus on grapevines. J Appl Ecol 34:878–888
Harmon J, Andow DA (2004) Indirect effects between shared prey: predictions for biological control. BioControl 49:605–626
Heithaus MR (2001) Habitat selection by predators and prey in communities with asymmetrical intraguild predation. Oikos 92:542–554
Holt RD (1977) Predation, apparent competition and structure of prey communities. Theor Popul Biol 12:197–229
Holt RD, Huxel GR (2007) Alternative prey and the dynamics of intraguild predation: theoretical perspectives. Ecology 88:2706–2712
Holt RD, Polis GA (1997) A theoretical framework for intraguild predation. Am Nat 149:745–764
Hosseini M, Ashouri A, Enkegaard A, Weisser WW, Goldansaz SH, Mahalati MN, Moayeri HRS (2010) Plant quality effects on intraguild predation between Orius laevigatus and Aphidoletes aphidimyza. Entomol Exp Appl 135:208–216
Janssen A, Montserrat M, HilleRisLambers R, Roos AMD, Pallini A, Sabelis MW (2006) Intraguild predation usually does not disrupt biological control. In: Brodeur J, Boivin G (eds) Trophic and guild interactions in biological control. Springer, The Netherlands, pp 21–44
Janssen A, Sabelis MW, Magalhaes S, Montserrat M, van der Hammen T (2007) Habitat structure affects intraguild predation. Ecology 88:2713–2719
Karban R, Hougen-Eitzmann D, English-Loeb G (1994) Predator-mediated apparent competition between two herbivores that feed on grapevines. Oecologia 97:508–511
Koss AM, Chang GC, Snyder WE (2004) Predation of green peach aphids by generalist predators in the presence of alternative, Colorado potato beetle egg prey. Biol Control 31:237–244
Letourneau DK, Jedlicka JA, Bothwell SG, Moreno CR (2009) Effects of natural enemy biodiversity on the suppression of arthropod herbivores in terrestrial ecosystems. Annu Rev Ecol Evol Syst 40:573–592
Lucas E, Demougeot S, Vincent C, Coderre D (2004) Predation upon the oblique-banded leafroller, Choristoneura rosaceana (Lepidoptera : Tortricidae), by two aphidophagous coccinellids (Coleoptera: Coccinellidae) in the presence and absence of aphids. Eur J Entomol 101:37–41
Madsen M, Terkildsen S, Toft S (2004) Microcosm studies on control of aphids by generalist arthropod predators: effects of alternative prey. BioControl 49:483–504
Messelink GJ, van Maanen R, van Steenpaal SEF, Janssen A (2008) Biological control of thrips and whiteflies by a shared predator: two pests are better than one. Biol Control 44:372–379
Messelink GJ, van Maanen R, van Holstein-Saj R, Sabelis MW, Janssen A (2010) Pest species diversity enhances control of spider mites and whiteflies by a generalist phytoseiid predator. BioControl 55:387–398
Messelink GJ, Bloemhard CMJ, Cortes JA, Sabelis MW, Janssen A (2011) Hyperpredation by generalist predatory mites disrupts biological control of aphids by the aphidophagous gall midge Aphidoletes aphidimyza. Biol Control 57:246–252
Müller CB, Brodeur J (2002) Intraguild predation in biological control and conservation biology. Biol Control 25:216–223
Nomikou M, Janssen A, Sabelis M (2003) Phytoseiid predators of whiteflies feed and reproduce on non-prey food sources. Exp Appl Acarol 31:15–26
Payne RW, Murray DA, Harding SA, Baird DB, Soutar DM (2010) GenStat for windows (13th edition) introduction. VSN International, Hemel Hempstead, UK
Polis GA, Myers CA, Holt RD (1989) The ecology and evolution of intraguild predation: potential competitors that eat each other. Annu Rev Ecol Syst 20:297–330
Ramakers PMJ (1989) Biological control in greenhouses. In: Minks AK, Harrewijn P (eds) Aphids, their biology, natural enemies and control. Elsevier, Amsterdam, The Netherlands, pp 199–208
Rosenheim JA (1998) Higher-order predators and the regulation of insect herbivore populations. Annu Rev Entomol 43:421–447
Rosenheim JA, Kaya HK, Ehler LE, Marois JJ, Jaffee BA (1995) Intraguild predation among biological control agents: theory and evidence. Biol Control 5:303–335
Sabelis MW, Janssen A, Lesna I, Aratchige NS, Nomikou M, van Rijn PCJ (2008) Developments in the use of predatory mites for biological pest control. IOBC/WPRS Bull 32:187–199
Shipp JL, Ramakers PMJ (2004) Biological control of thrips on vegetable crops. In: Heinz KM, van Driesche RG, Parrella MP (eds) Biocontrol in protected culture. Ball Publishing, Batavia, USA, pp 265–276
Snyder WE, Ives AR (2001) Generalist predators disrupt biological control by a specialist parasitoid. Ecology 82:705–716
Snyder WE, Ives AR (2003) Interactions between specialist and generalist natural enemies: parasitoids, predators, and pea aphid biocontrol. Ecology 84:91–107
Symondson WOC, Sunderland KD, Greenstone MH (2002) Can generalist predators be effective biocontrol agents? Annu Rev Entomol 47:561–594
Tommasini MG, van Lenteren JC, Burgio G (2004) Biological traits and predation capacity of four Orius species on two prey species. Bull Insectol 57:79–93
van den Meiracker RAF, Ramakers PMJ (1991) Biological control of the western flower thrips Frankliniella occidentalis, in sweet pepper, with the anthocorid predator Orius insidiosus. Meded Fac Landbouww Rijksuniv Gent 56:241–249
van Lenteren JC (2000) A greenhouse without pesticides: fact or fantasy? Crop Prot 19:375–384
van Lenteren JC, Woets J (1988) Biological and integrated pest control in greenhouses. Annu Rev Entomol 33:239–269
van Steenis MJ (1993) Intrinsic rate of increase of Aphidius colemani Vier. (Hym., Braconidae), a parasitoid of Aphis gossypii Glov. (Hom., Aphididae), at different temperatures. J Appl Entomol 116:192–198
Vance-Chalcraft HD, Rosenheim JA, Vonesh JR, Osenberg CW, Sih A (2007) The influence of intraguild predation on prey suppression and prey release: a meta-analysis. Ecology 88:2689–2696
Yoo HJS, O’Neil RJ (2009) Temporal relationships between the generalist predator, Orius insidiosus, and its two major prey in soybean. Biol Control 48:168–180
Acknowledgments
This study was funded by the Dutch Ministry of Agriculture, Nature and Food Quality. We thank L. Kok and E. de Groot for assistance in the greenhouse and laboratory experiments. Koppert Biological Systems and Biobest NV are thanked for supplying the natural enemies. Comments by two anonymous reviewers and Patrick De Clercq substantially improved the manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Patrick De Clercq
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Messelink, G.J., Bloemhard, C.M.J., Sabelis, M.W. et al. Biological control of aphids in the presence of thrips and their enemies. BioControl 58, 45–55 (2013). https://doi.org/10.1007/s10526-012-9462-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-012-9462-2