Abstract
Several studies have shown that biological control of pests can be improved by supplying extra food to natural enemies. This increases population levels of the enemies, resulting in decreases in pest densities. In theory, however, supplying food can also have negative effects on biological control. We specifically tested for such negative effects, using a predator–prey system consisting of the whitefly Bemisia tabaci (Gennadius) and a predatory mite Amblyseius swirskii (Athias-Henriot). This predator attacks eggs and young instars of the whitefly, but also feeds on pollen. We added pollen to populations of predators and whiteflies on isolated cucumber plants. Although the set-up of our experiments would favour the occurrence of a negative effect of the addition of pollen on biological control, we found increased control throughout the experiment. This shows that the control of whiteflies by A. swirskii can be improved by supplementing the predators with pollen.
Similar content being viewed by others
Introduction
There is by now a substantial body of theory on interactions between prey populations that are attacked by a common predator population. One of these interactions is called apparent competition (Holt 1977) because increases in numbers of one prey population have a negative effect on equilibrium numbers of the other prey population (Holt 1977; Chaneton and Bonsall 2000). Hence, the outcome of the interaction resembles competition between the two prey species, but is caused through the interaction with the shared predator. Subsequent theory has shown that sharing a predator can also lead to positive effects in the short term (Holt and Kotler 1987; Abrams and Matsuda 1993; Holt and Lawton 1994; Abrams et al. 1998). This so-called apparent mutualism can occur when an increase in density of one prey leads to predator satiation, resulting in lower predation rates on the other prey species (Holt and Lawton 1993; Holt and Lawton 1994), when time available for handling prey is limited (Holt 1977), or when predators show switching behaviour (Abrams and Matsuda 1993; Abrams and Matsuda 1996). In addition, long-term positive effects can occur when the predator has a functional response that levels off at higher prey densities and when populations cycle (Holt 1997; Abrams et al. 1998). Hence, depending on the time scale and on the type of dynamics, theory predicts that a shared natural enemy can generate positive or negative indirect interactions between prey species.
Various researchers have shown that addition of non-prey food for predators can result in improved control of pests (Collyer 1964; Karban et al. 1994; Hanna et al. 1997; Walde et al. 1997; Liu et al. 2006). The mechanism causing this is similar to that of apparent competition; the addition of non-prey food results in an increase of predator numbers, which results in a decrease of pest densities. As in apparent competition, the possibility that addition of non-prey food for predators may also result in decreased control, at least in the short term, has not received much attention in the biological control literature (van Rijn et al. 2002). However, this is especially relevant for biological control systems that exist for a limited period, where the dynamics of pests and natural enemies are often transient (van Veen et al. 2006). We studied the effect of the addition of non-prey food on the transient dynamics of an arthropod predator–prey system. We specifically studied a system in which the conditions favour negative effects on biological control.
The study system consisted of the whitefly Bemisia tabaci (Gennadius) and its predatory mite Amblyseius swirskii (Athias-Henriot) (Zannou et al. 2007). The predator reproduces and develops when feeding on whitefly immatures (B. tabaci and Trialeurodes vaporariorum (Westwood)) but also on pollen and Western flower thrips (Frankliniella occidentalis (Pergande)) as a food source (Nomikou et al. 2001; Nomikou et al. 2003; Messelink et al. 2006; Messelink et al. 2008). In a greenhouse, A. swirskii was found to suppress whitefly populations on single cucumber plants when pollen was supplied to the predators every week (Nomikou et al. 2002). It is still unclear, however, how the addition of pollen affected the population dynamics of the prey, since no controls without this non-prey food were performed (Nomikou et al. 2002).
Several characteristics of the experimental system favour the occurrence of negative effects of the addition of food on whitefly control. First, the growing season of greenhouse crops is short; hence, the dynamics of whiteflies and predatory mites are transient. Second, the functional response of the predatory mites is likely to be of type II (Sabelis 1992; Sabelis and van Rijn 1997), which might lead to positive indirect effects between pollen and whitefly numbers (Holt 1977; Abrams and Matsuda 1993). Third, in the experiments described below, we supplied pollen in small plastic vials that were suspended from one of the leaf stems, whereas the prey reside mainly on the underside of all leaves. Thus, predators did not encounter prey and pollen simultaneously, forcing the predators to switch from consuming pollen to consuming prey, which can also result in apparent mutualism (Abrams and Matsuda 1993).
Materials and methods
Cultures
Cucumber plants (var. Ventura RZ®, RijkZwaan, De Lier, The Netherlands) were grown in pots (2 l) in a greenhouse (25°C; l:d = 16:8) until three weeks old. Bemisia tabaci strain B (J.J. Fransen, personal communication) was obtained from the Research Station for Floriculture in Aalsmeer in March 1995, where it was cultured on poinsettia. We cultured this whitefly strain on cucumber plants in climate boxes (27°C; 16 h light).
Amblyseius swirskii was collected in Israel (location Revadim) in 1997 on cotton infested with B. tabaci (Nomikou et al. 2001). It was cultured on plastic arenas (8 × 15 cm) placed on a wet sponge in a plastic tray with water (see Overmeer 1985). Strips of wet tissue were placed on the plastic arena along its periphery so that the predators had access to water. Glue barriers were applied on this tissue to prevent escape and contamination with other mite species. A piece of transparent plastic sheet (1–2 cm2), folded in the shape of a roof, was placed on each arena and functioned as a shelter for the mites (Overmeer 1985). A few cotton threads were put underneath the shelter to serve as oviposition substrate (Overmeer 1985). Broad bean pollen (Vicia faba L.) was offered as food for the predators by dusting it on the arenas twice per week. Broad bean pollen was collected from plants cultivated in a greenhouse compartment at the University of Amsterdam and cattail pollen, Typha latifolia L. sp., was collected at the university campus. Both types of pollen were kept at −20°C before being fed to the predators. The predatory mite culture was maintained in a climate room (25°C, 60% R.H.). In order for the predators to become accustomed to the greenhouse conditions, we started separate rearing arenas in the greenhouse with individuals from the predator culture, where they were fed cattail pollen. All mites used in the experiments originated from these greenhouse cultures.
Experimental set-up
Population experiments were carried out in cages in a greenhouse (25°C, 60% RH, 16 h photoperiod). The cages (0.8 × 0.8 × 1 m) consisted of a metal frame, a plastic bottom, a Plexiglas top and three sides with insect and mite proof gauze (mesh 80 μm). A Plexiglas door covered the fourth side and closed with strips of magnetic tape. The cages were placed on four tables, each table consisting of a tray filled with a 2–3 cm layer of water. The cages were suspended above the water by placing them on bricks. In this way, a water barrier underneath the cages prevented escape and invasion of mites. Temperature and humidity loggers recorded the conditions in each cage at 30-min intervals.
Three cages were placed on each table. One potted cucumber plant of three weeks old was placed in each of the cages. Three wooden sticks (90 cm long) were stuck in the soil around the young plant and were tied together near the tip, thus forming a tent-like frame that supported the plant. Fertilizer was supplied twice per week via the irrigation water (N:P:K = 28:14:14). Plants were allowed to flower and to grow lateral stems and fruits were removed when full-grown. Control cages received whiteflies only. All other cages received predators and whiteflies; Typha sp. pollen was supplied to half of the plants.
Initial conditions and pollen supply
Twenty adult whiteflies (10 females and 10 males) were introduced on each of the 12 plants. Three days later, each plant (except for controls) was supplied with predators, 48 females and 120 juveniles plus males on the 3rd lowest leaf of all plants, using a fine brush. One day later, the number of adult female predators that was found on the plants was always lower than 40 due to escapes. To recreate equal initial conditions we added females from the cultures to achieve a fixed initial number of 40 on all plants.
One day after predator release, half of the plants were supplied with 25–30 mg of Typha sp. pollen in a plastic vial (19 mm diameter and 15 mm high) suspended from the base of the stem of the 3rd leaf from below by means of a piece of electric wire, which was inserted in two holes at opposite sides near the rim of the vial (hence, the vial resembled a miniature bucket). To create control plants that differed from the treatment only by pollen being absent, we suspended empty vials from corresponding leaf stems on the pollen-free plants. Three days later (one week after the first whitefly release), we introduced another ten pairs of adult whiteflies.
Every week, plants that received pollen were supplied with 25–30 mg of fresh pollen in a new vial that was attached two leaf stems higher than the previous vial. This quantity of pollen is sufficient to sustain a population of phytoseiids in laboratory cultures (M. Nomikou, pers. obs.). Moreover, the quality of Typha sp. pollen remains good for at least one week (Nomikou et al. 2002; van Rijn et al. 2002). Vials with pollen were removed from the plant after three weeks and checked for predators with a binocular microscope. Predators were transferred back to the leaf that had been closest to the vial. Hence, plants provided with pollen carried one vial in the first week, two vials in the second week, and three vials during the subsequent experimental period.
As a control, we followed the dynamics of whitefly populations without predators on six cucumber plants. Due to limited greenhouse space and the limited availability of cages, this control treatment could not be started at the same time as the predator treatments. We therefore started this control treatment right after the first cages became available from the predator treatment as a consequence of the destructive method used to sample the populations (at day 39 of the replicates with predators, see below). Cages were cleaned with alcohol to kill any remaining insects and mites before re-use for the control treatment. Plants were handled as above, and whiteflies were released as above. Moreover, plastic vials were attached to the same leaves as above, but they were not provided with pollen (whiteflies do not consume pollen).
Population dynamics
Adult whiteflies were counted while removing them from the plants with an aspirator. Subsequently, all leaves were detached from the plant and adult female predatory mites were counted by visual inspection of the detached leaf. Finally, four leaf discs (15 mm diameter each) were punched from each detached leaf, two from the left and two from the right half. These discs were stored in a closed plastic vial (3 cm diameter, c. 4 cm high) filled with 70% alcohol and the number of immature whiteflies on them was counted later. The number of instars was summed per leaf (four leaf discs) and the average per leaf was calculated subsequently. Individuals were classified in one of four classes: (1) eggs, (2) crawlers and second instars, (3) third instars and (4) pupae. The first destructive sampling was done 37–39 days after the start of the experiment, the second on day 58–59. For reasons of time limitation, adult whitefly populations were counted on four control plants only (i.e. without predators), three on the 38–39th day after the first whitefly release and one on the 59th day after the first whitefly release. The numbers of adult whiteflies and predators on the remaining two plants were estimated to an order of magnitude (100, 1,000 or 10,000), and juveniles were not counted.
Because pollen was supplied in vials near a few leaves at positions specified above, predators could aggregate on these leaves and prey could avoid these leaves. To test whether such a distribution occurred, we compared the fraction of the total numbers of predators and prey on the leaves closest to the vials with or without pollen.
Data analysis
Temperature conditions during the experiment varied over time, but did not differ among the cages at any given time. The average temperature was around 25°C (range 18–33°C). Humidity varied both over time and among the cages. Even though the positions of each of the two treatments were randomised and the control experiments were carried out in cages formerly occupied by either of the two treatments, there appeared to be a systematic gradient in humidity: c. 50% in the control experiments, c. 60% in the predator treatment without pollen and c. 65% in the treatment with predators and pollen. For this reason, we initially included humidity as a covariate in the analysis, but it proved not to be significant and was therefore removed from further analysis. The numbers of adult whiteflies and predators were analysed with a generalized linear model with quasi-Poisson error distribution to correct for overdispersion (Crawley 2007). Densities of the juvenile whitefly stages were log-transformed and analysed with ANOVA. The fraction of adult whiteflies, adult predators and immature whiteflies on the leaves with vials was analysed with an ANOVA on the arcsine-square root transformed proportions. Treatments were contrasted through model simplification (Crawley 2007). All statistical analyses were done with R (R Development Core Team 2006). Because the control treatment without predators was not done during exactly the same period as the two treatments with predators, it cannot be compared to the two treatments with predators. We therefore refrained from including it in the statistical analysis, but the data are presented to give an impression of the effects of the predators on whitefly dynamics. This does not impede our study in any way, because we were mainly interested in the effect of predators on whitefly dynamics in the absence and presence of pollen, and these two treatments were done simultaneously.
Results
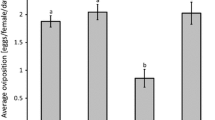
Plants to which pollen was supplied had slightly more leaves than plants without pollen (average ± SE pollen: 61.7 ± 2.1, no pollen: 55.0 ± 3.2, respectively), but this difference was not significant (Wilcoxon rank sum test). Adult whitefly populations per plant in the predator treatments increased on average 1.8 times in presence of pollen, 14-fold in absence of pollen, and increased exponentially to 350 times the initial numbers in the controls (i.e. without predators) (Fig. 1). The number of adult whiteflies differed significantly among the two treatments with predators and with time, but the interaction of treatment with time was not significant (Table 1). This suggests a negative effect of the presence of pollen on the numbers of whiteflies (Fig. 1).
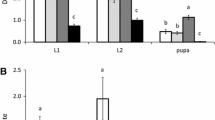
The density of most immature whitefly stages also differed significantly between treatments with and without pollen as well as with time (Table 2), the exception being third instars, which did not differ significantly between the treatment with or without pollen. Except for the third instars, there was also a significant interaction of time and the presence or absence of pollen (Table 2). The density of most immature stages was lower in the presence than in the absence of pollen, especially after 59 days, thus confirming a negative effect of pollen on whitefly numbers (Fig. 2).
Significantly more predators were present on the plants with pollen than on plants without pollen (Table 3). The average numbers of predators per plant in the presence of pollen increased from 40 one day after release to 225 at the first sampling date and then decreased slightly to 215 at the second sampling date. In the absence of pollen, the numbers of predators remained more constant (45.7 and 59.0 on the 1st and 2nd sampling date, respectively). There was no significant effect of time (Table 3).
In the treatments with pollen, a slightly larger fraction of the predator population was found on the leaves close to the vials with pollen than on similar leaves in the treatments without pollen (Fig. 3, F 1,4 = 5.0, P = 0.089). The fraction of adult and immature prey on the leaves close to the vials did not differ between the treatment with and without pollen (Fig. 3, adults: F 1,4 = 1.50, P = 0.29, immatures: F 1,4 = 1.56, P = 0.28).
The fraction of adult predators and adult and immature whiteflies on leaves close by small vials with or without pollen after 59 days. Plants were either supplied with pollen in small vials (pollen) or received empty vials (no pollen). Shown are average fractions (+SE) of the total numbers that were found on a plant
Discussion
Whitefly populations increased exponentially in the absence of predatory mites, but their growth was substantially reduced in the presence of predators. Addition of pollen as alternative food for the predatory mites resulted in a further reduction of whiteflies. Populations of predators reached higher numbers on plants with pollen than on plants without, whereas prey density (eggs and young immature whiteflies) on plants with pollen was lower than on plants without pollen. All these effects are in agreement with the concept of apparent competition, where the density of a prey type is negatively affected by the presence of another food type through their joint effect on a shared predator population.
Although the conditions of our experiments were such that any indirect effects of the addition of food to a predator–prey system would yield negative effects on biological control, we found no evidence for this. These conditions were (1) a relatively short experimental period with non-equilibrium dynamics; (2) a type II functional response of the predatory mites, which might also lead to negative effects of pollen on whitefly control (Holt 1977; Abrams and Matsuda 1993); (3) the spatial separation of the two food types, which should result in switching of the predators (Abrams and Matsuda 1993). With respect to the first condition, it is possible that negative effects on the control of whiteflies were present at an even shorter time scale than was studied here; the addition of pollen can initially result in satiation of the predators present, thus decreasing the predation rate on the prey (Holt and Lawton 1993; Holt and Lawton 1994). If this effect did occur in our experiment, it might have gone undetected because of the increased numerical response of the predator population, even before the first sampling date. Nevertheless, our results show that the addition of alternative food for predators does not result in decreased, but rather increased control at a temporal scale relevant for crop protection.
With respect to the second condition, laboratory tests with ample supply of crawlers of B. tabaci showed that the addition of pollen neither altered the predation rate nor increased the oviposition rate of the predators (Nomikou et al. 2004), but this was with pollen and prey in the same area. In contrast, experiments with another species of whitefly, T. vaporariorum, and an alternative prey, the Western flower thrips, showed that A. swirskii consumed half the number of whitefly eggs and half the number of thrips larvae when offered together than when offered separately (Messelink et al. 2008). Such reduced predation of whiteflies would certainly result in a negative effect on the biological control of whiteflies, at least in the short term. However, this was compensated by an increased numerical response of predators on a mixed diet (Messelink et al. 2008).
As far as the third condition is concerned, the spatial separation of pollen and prey in the experiments described here did not result in negative effects of the addition of pollen on whitefly control, despite the fact that it will have cost the predators time and energy to commute from the pollen patches to leaves with whiteflies. Few immature vulnerable whiteflies were found on the leaves close to the vials with pollen (Fig. 3), and this will have forced predators to commute between pollen vials and leaves with vulnerable stages of the prey. Although the distance between vials with pollen and the top leaves of plants are relatively large for the small (<1 mm), wingless and blind predatory mites, this spatial segregation of pollen and prey did not result in a significant difference in distribution of predators or whiteflies on plants with pollen compared to plants without pollen. This suggests that the local supply of pollen on the plant did not arrest the predatory mites to such an extent that they failed to find and consume prey in other strata on the plant. We therefore conclude that the addition of pollen to the crop resulted in increased biological control of whiteflies, even when the pollen is supplemented in a concentrated form.
Van Rijn et al. (2002) describe experiments similar to ours with a different predator–prey system: another species of predatory mite, Iphiseius degenerans (Berl.), and another prey species, the Western flower thrips. Their system has as additional peculiarity that the prey can also feed and reproduce on pollen; hence, this would be an extra reason for finding negative effects of the addition of pollen on the control of thrips. However, they also did not find such a negative effect.
Van Rijn et al. (2002) show that biological control can be improved by providing alternative food to predators. Here, we also show that predators reduced adult whitefly densities by a factor 28 in absence of pollen, but the addition of pollen resulted in a further eightfold decrease of adult whitefly densities (Fig. 1). In a study with the same species of predator as used here, Messelink et al. (2008) show that addition of another prey species of the predatory mite also resulted in better control of whiteflies. However, the alternative prey used in the latter study, Western flower thrips, is a pest itself. Hence, strategies to use this alternative prey to increase pest control of whiteflies are bound to be risky. Pollen, the supplemental food used here, does not pose such a risk. We therefore suggest that the addition of pollen or other non-prey food to a crop is a viable strategy to increase biological control of whiteflies.
References
Abrams PA, Matsuda H (1993) Effects of adaptive predatory and anti-predator behaviour in a two-prey-one-predator system. Evol Ecol 7:312–326
Abrams PA, Matsuda H (1996) Positive indirect effects between prey species that share predators. Ecology 77:610–616
Abrams PA, Holt RD, Roth JD (1998) Apparent competition or apparent mutualism? Shared predation when populations cycle. Ecology 79:201–212
Chaneton EJ, Bonsall MB (2000) Enemy-mediated apparent competition: empirical patterns and the evidence. Oikos 88:380–394
Collyer E (1964) The effect of an alternative food supply on the relationship between two Typhlodromus species and Panonychus ulmi (Koch) (Acarina). Entomol Exp Appl 7:120–124
Crawley MJ (2007) The R Book. Wiley, Chichester
Hanna R, Wilson LT, Zalom FG, Flaherty DL (1997) Effects of predation and competition on the population dynamics of Tetranychus pacificus on grapevines. J Appl Ecol 34:878–888
Holt RD (1977) Predation, apparent competition, and structure of prey communities. Theor Popul Biol 12:197–229
Holt RD (1997) Community modules. In: Gange AC, Brown VK (eds) Multitrophic interactions in terrestrial systems. British Ecological Society, Blackwell, pp 333–350
Holt RD, Kotler BP (1987) Short-term apparent competition. Am Nat 130:412–430
Holt RD, Lawton JH (1993) Apparent competition and enemy-free space in insect host-parasitoid communities. Am Nat 142:623–645
Holt RD, Lawton JH (1994) The ecological consequences of shared natural enemies. Annu Rev Ecol Syst 25:495–520
Karban R, Hougen-Eitzman D, English-Loeb G (1994) Predator-mediated apparent competition between herbivores that feed on grapevines. Oecologia 97:508–511
Liu C-Z, Yan L, Li H-R, Wang G (2006) Effects of predator-mediated apparent competition on the population dynamics of Tetranychus urticae on apples. BioControl 51:453–463
Messelink GJ, van Steenpaal SEF, Ramakers PJM (2006) Evaluation of phytoseiid predators for control of western flower thrips on greenhouse cucumber. BioControl 51:753–768
Messelink GJ, van Maanen R, van Steenpaal SEF, Janssen A (2008) Biological control of thrips and whiteflies by a shared predator: two pests are better than one. Biol Control 44:372–379
Nomikou M, Janssen A, Schraag R, Sabelis MW (2001) Phytoseiid predators as potential biological control agents for Bemisia tabaci. Exp Appl Acarol 25:271–291
Nomikou M, Janssen A, Schraag R, Sabelis MW (2002) Phytoseiid predators suppress populations of Bemisia tabaci on cucumber plants with alternative food. Exp Appl Acarol 27:57–68
Nomikou M, Janssen A, Sabelis MW (2003) Phytoseiid predators of whiteflies feed and reproduce on non-prey food sources. Exp Appl Acarol 31:15–26
Nomikou M, Janssen A, Schraag R, Sabelis MW (2004) Vulnerability of Bemisia tabaci immatures to phytoseiid predators: consequences for oviposition and influence of alternative food. Entomol Exp Appl 110:95–102
Overmeer WPJ (1985) Rearing and handling. In: Helle W, Sabelis MW (eds) Spider mites, their biology, natural enemies and control, vol 1b. Elsevier, Amsterdam, pp 162–170
R-Development-Core-Team (2006) R: a language and environment for statistical computing, 2.3.1 edn. R Foundation for Statistical Computing, Vienna
Sabelis MW (1992) Predatory arthropods. In: Crawley MJ (ed) Natural enemies: the population biology of predators, parasites and diseases. Blackwell, Oxford, pp 225–264
Sabelis MW, van Rijn PCJ (1997) Predation by insects and mites. In: Lewis T (ed) Thrips as crop pests. CAB International, London, pp 259–354
van Rijn PCJ, van Houten YM, Sabelis MW (2002) How plants benefit from providing food to predators even when it is also edible to herbivores. Ecology 83:2664–2679
van Veen FJF, Memmott J, Godfray HCJ (2006) Indirect effects, apparent competition and biological control. In: Brodeur J, Boivin G (eds) Trophic and guild interactions in biological control. Springer, Dordrecht, pp 145–170
Walde SJ, Hardman JM, Magagula CN (1997) Direct and indirect species interactions influencing within-season dynamics of apple rust mite, Aculus schlechtendali (Acari: Eriophyidae). Exp Appl Acarol 21:587–614
Zannou ID, De Moraes GJ, Ueckermann EA, Oliveira AR, Yaninek JS, Hanna R (2007) Phytoseiid mites of the subtribe Amblyseiina (Acari: Phytoseiidae: Amblyseiini) from sub-Saharan Africa. Zootaxa 1550:1–47
Acknowledgments
We thank Paul van Rijn for advice. Ludek Tikovsky and Harold Lemereis were most helpful in arranging greenhouse space and Rijk Zwaan B.V., De Lier provided cucumber seeds. Eric van Gool, Sara Magalhães and Marta Montserrat are thanked for discussions. An earlier version of the manuscript was improved by J.C. van Lenteren and J. Fransen. Comments by two anonymous reviewers and Patrick De Clercq substantially improved the ms. The research was funded as project ABI. 4165 by the Technology Foundation STW to MN and AJ. The experiments described in this paper comply with the current laws of the country in which the experiments were performed.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Patrick De Clercq
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Nomikou, M., Sabelis, M.W. & Janssen, A. Pollen subsidies promote whitefly control through the numerical response of predatory mites. BioControl 55, 253–260 (2010). https://doi.org/10.1007/s10526-009-9233-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-009-9233-x