Abstract
Silver nanoparticles are the most important nanomaterials for antibacterial uses and are famous for their strong inhibitory and antibacterial effects. In recent years, extensive studies have been undertaken on the use of antimicrobial properties of silver, incorporated within aquaculture industry. To evaluate the scientific basis for the use of the nano silver in shrimp aquaculture, in this study the antimicrobial activities of colloidal nano silver with two different sizes (16.62 and 22.22 nm) was evaluated against gram negative bacteria, V. harveyi. Before the experiments, cAgNPs were characterized using several analytical techniques. Well diffusion method, micro-dilution tests (MIC and MBC) and kinetic of death were used to evaluate the bactericidal activity of the nanoparticles. Results showed that MIC and MBC values of cAgNPs in both studied sizes are equal (MIC = MBC). Best bactericidal kinetics in the presence of 16.62 and 22.22 nm nanoparticles obtained at 4 and 6 h, respectively. The obtained results suggested that smaller silver nanoparticles had a faster antibacterial activity than the larger particles. According to the obtained results, the activity of cAgNPs against V. harveyi is fast and has potential for the treatment of bacterial infection in aquaculture.
Similar content being viewed by others
Introduction
In past decades, shrimp culture has developed throughout the world. Over the past decade, the shrimp industry has been accompanied by the occurrence of serious bacterial diseases such as vibriosis (especially the luminous V. harveyi) (Khamesipour et al. 2014). V. harveyi is known as an important causative agent of vibriosis disease in shrimp farms worldwide, which causes mortalities up to 100 % at a time in shrimp rearing systems (Ramesh et al. 2014). It is worth noting that Vibrio harveyi is a species of gram negative, bioluminescent, facultative anaerobic and halophilic bacteria in the genus Vibrio (Khamesipour et al. 2014; Ramesh et al. 2014). During the recent several years, researchers have reported that use of antibiotics for controlling disease outbreaks may lead to the emergence of antibiotic resistance bacteria (Domrongpokkaphan and Wanchaitanawong 2006). One of the alternative methods for controlling resistance of bacteria in treatment of diseases is the use of nanomaterials such as silver nanoparticles (AgNPs) (Kim et al. 2007; Petrus et al. 2011). Among the nanoparticles, silver or its compounds have known to have high antimicrobial and broad spectrum antimicrobial activities for bacteria, fungi and virus (Cho et al. 2005; Petrus et al. 2011). Compared with the eukaryote cells (or mammalian cells), AgNPs exhibits higher toxicity to microorganisms (Li et al. 2010). The antimicrobial properties of AgNPs are well established, and several mechanisms for their bactericidal effects have been proposed (Sondi and Salopek-Sondi 2004). Recent studies using silver nanoparticles have demonstrated a broad range of antimicrobial activity against several gram positive pathogenic bacteria such as Staphylococcus aureus (Kim et al. 2007; Petrus et al. 2011), B. subtilis (Savithramma et al. 2011) and Lactococcus garvieae (Soltani et al. 2009), gram negative pathogenic bacteria such as Klebsiella pneumonia (Savithramma et al. 2011), Salmonella typhi (Petrus et al. 2011), V. cholera (Petrus et al. 2011; Dibrov et al. 2002), V. fischeri (Binaeian et al. 2012), V. harveyi (Vaseeharan et al. 2010), V. alginolyticus (Vaseeharan et al. 2012), Proteus vulgaris (Savithramma et al. 2011), E. coli (Petrus et al. 2011; Li et al. 2010; Savithramma et al. 2011; Kim et al. 2007; Sondi and Salopek-Sondi 2004), Pseudomonas aeroginosa (Savithramma et al. 2011), Streptococcus iniae (Soltani et al. 2009), Yersinia ruckeri (Soltani et al. 2009), Aeromonas hydrophila (Sarkar et al. 2012; Soltani et al. 2009), viruses, pathogenic fungi (Johari et al. 2015) and eukaryotic microorganisms (Vaseeharan et al. 2012).
So far, several investigations have described different parameters that influence antibacterial effects of AgNPs. These factors include properties such as size, shape, surface chemistry, crystallinity, capping agent and dose of AgNPs (Martínez-Castañón et al. 2008; Zhang et al. 2014). However, the size of nanoparticles is one of the most important factors determining antimicrobial potential of AgNPs. Furthermore, the main objective of the present study was to evaluate the effect of particle size on the antibacterial activity of AgNPs against Vibrio harveyi. Therefore, the antibacterial activity of AgNPs with two different sizes (16.62 and 22.22 nm) was assessed by determining the minimal inhibitory concentration (MIC), the minimum bactericidal concentration (MBC), well diffusion method and measuring the dynamic growth curve of the bacteria.
Materials and methods
Silver nanoparticles characterization
The colloidal silver nanoparticles (cAgNPs), type L (commercial name: Nanocid®), were prepared from Nano Nasb Pars Co. Ltd., Tehran, Iran. The present research used two sizes of colloidal silver nanoparticles. The colloid product was synthesized using a novel process involving the photo-assisted reduction of Ag+ to metallic nanoparticles, registered under United States Patent Application No: 20090013825 (Rahman Nia 2011). Before launching the experiments, cAgNPs were assayed by different methods: To confirm the presence of Ag nanoparticles in colloidal solutions, their UV–visible absorption spectra were recorded using an UV–visible double beam spectrophotometer (UV-1601, Shimadzu, Japan), between 200 and 800 nm. The crystalline structure of Ag nanoparticles was characterized using a X-ray diffractometer (Bruker D8 Advance, Bruker AXS, Germany) employing Cu Kα radiation (λ = 1.54 Å) at 2θ ranges from 10° to 80°. Average particle sizes and polydispersity indices (PDI or heterogeneity index) of cAgNPs were measured by a dynamic light scattering (DLS) technique using Zetasizer Nano ZS90 (Malvern Instruments, Malvern, UK) at 25 °C. Concentration of Ag in Ag nanoparticles was determined with inductively coupled plasma atomic emission spectroscopy (ICP–OES Perkin-Elmer Optima, 5300 DV, USA).
Microorganism and media
The strain used in this research was luminescence V. harveyi IS01PTTC1755 (NCBI: GU974342.1), which had been isolated from diseased shrimp of Bushehr province farms, identified in Iran Scientific and Industrial Research Organization (PTCC: 1755) and documented in the American Gene Bank (NCBI: GU974342.1).
All culture medium contained Mueller-Hinton Broth (MHB), Mueller-Hinton agar (MHA), Nutrient broth (NB), Tryptic soy agar (TSA) and Tryptic soy broth (TSB) and sodium chloride were purchased from Merck (Germany).
MIC and MBC determination
MICs of the cAgNPs were determined by the serial dilution technique using Mueller-Hinton broth (supplemented with 2.5 % NaCl) as described by Emmanuel et al. (2015), with slight modifications. Briefly, about 1 mL of Mueller-Hinton broth medium was added to 12 test tubes. Final concentrations of the cAgNPs were adjusted between 1/2 in the first tube and 1/1024 in the tenth tube. Then 1 mL of bacterial suspension containing 1.5 × 108 CFU/mL (0.5 McFarland unit) was added to all tubes and incubated at 30 °C for 24 h. After incubation, first dilution with no visible growth was considered as MIC of the cAgNPs against V. harveyi. Tubes containing nanoparticles and without nanoparticles were used as controls.
MBC was determined by spread plating 100 μL of all clear tubes (no visible growth) in the MIC assay on Tryptic soy agar (supplemented with 2.5 % NaCl). Then, plates were incubated at 30 °C for 24 h. After incubation period, the highest dilution which inhibited colony formation on agar was noted as MBC.
Kinetics study of cAgNPs against Vibrio harveyi
The kinetic study was carried out using protocol of Chatterjee et al. (2011) with minor modifications. Briefly, 5 % of overnight broth culture of V. harveyi (50 mL with ODA600 = 0.2 equivalent to 3 × 108 CFU/mL) was mixed with fresh nutrient broth (100 mL) supplemented with AgNPs at dose equivalent to MIC (~4 mg/L). The cultures were incubated at 30 °C with shaking at 150 rpm. The microbial growth was monitored by measuring the optical density (O.D) at 600 nm at 30-min intervals for 7 h using UV–Vis Spectrophotometer (6800 Jenway Inc., England).
Zone of inhibition (ZOI) assay
The antibacterial activity of cAgNPs was assayed against Vibrio harveyi by using agar well diffusion method (Ahmad and Beg 2001). In brief, Mueller-Hinton Agar (MHA) plates were inoculated uniformly with 1.5 × 108 CFU/mL (0.5 McFarland Standard) of bacterial suspension, using sterile swabs in triplicates. Wells of 6 mm size were created with sterile cork borer in the agar plates containing the bacterial inoculum. Then, 50 μL of cAgNPs was introduced into each of the wells and allowed to diffuse at 4 °C for 2 h. Finally, the plates were incubated at 30 °C for 24 h.
Results
Particle characterization of cAgNPs
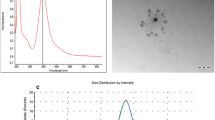
Silver nanoparticles existence in tow suspensions (cAgNPs) were confirmed using UV–Vis spectral analysis and both samples showed silver surface plasmon resonance (SPR) band at 420 nm (Fig. 1). The crystalline structure of Ag-NPs in tow colloids was confirmed by X-ray diffraction analysis as shown in Fig. 2a, b. As shown in Fig. 2, there was no obvious difference in the XRD patterns between 16.62 and 22.22 nm silver nanoparticles. Four distinct diffraction peaks were observed for AgNPs at (111), (200) (220) and (311) (Fig. 2). These peaks are exactly matched with the Joint Committee on Powder Diffraction Standards (JCPDS) file No. 04-0783. Particle size distribution and polydispersity index (PDI) of silver nanoparticles in two colloids were obtained by Dynamic light scattering (DLS) technique (Fig. 3 a and b). The DLS results indicated that the mean sizes (Z-average) of nanoparticles were 16.62 and 22.22 nm, and the mean of PDI of the nanoparticles were 0.23 and 0.21, respectively. The concentrations of Ag nanoparticles were measured by ICP_OES and were found to be approximately 4000 ppm (in both colloids).
Determination of MIC and MBC
The results of the MIC and MBC of cAgNPs (with different sizes) against V. harveyi are shown in Table 1. The MIC and MBC values of both sizes of cAgNPs were equal (MIC16.6 and 22.22 nm = MBC16.62 and 22.22 nm) (3.91 mg/L). The calculation of MIC/MBC ratio shows that this value is equal to 1 for V. harveyi (Table 1).
Kinetics of antibacterial activity
The study of the effects of cAgNPs on growth kinetics of V. harveyi was done in comparison with the normal growth (Fig. 4). A very strong antimicrobial activity was seen by both cAgNPs. However, the fastest antibacterial activity was seen by 16.62 nm AgNPs. As shown in Fig. 4, 16.62 nm AgNPs reduced bacterial growth to undetectable levels after 4 h of contact, whereas in 22.22 nm AgNPs the bacteria were killed after 6 h incubation (almost all treated bacterial cells were dead).
Well diffusion studies
Besides MIC, MBC and growth kinetics methods, antibacterial activity of cAgNPs was assessed using the well diffusion technique. The both cAgNPs exhibited excellent inhibitory effects against V. harveyi (Fig. 5). And ZOI for sizes of 22.22 and 16.62 nm were observed 42.1 ± 0.2 mm and 43.12 ± 0.14 mm, respectively. But there was insignificant difference between antibacterial activities of different sizes of cAgNPs against tested bacteria (P > 0.05).
Discussion
In recent times emergence of drug resistance in microbial species (largely bacteria, such as Vibrio sp.) have become a serious concern in aquaculture industry (Vaseeharan et al. 2012). Therefore, to deal with this challenge (bacterial resistance) new therapeutic strategies, such as nanoparticles, can be useful. The use of AgNPs as antibacterial agent is relatively new, and the antibacterial potential of silver nanoparticles has already been proved by many researchers (Morones et al. 2005; Kim et al. 2007; Sanpui et al. 2008; Martínez-Castañón et al. 2008; Rai et al. 2009; Lago et al. 2011; Chudobova et al. 2013; Zhong et al. 2013; Ivask et al. 2014). The aim of present study was to assess the antibacterial effects of two different sizes of cAgNPs against V. harveyi. The dilution method results indicated that both sizes of cAgNPs have robust antibacterial activity (see Table 1), and MIC and MBC values of both size of cAgNPs were equal (MIC = MBC). Above result strongly showed that cAgNPs has bactericidal properties. Antimicrobial assay by growth kinetics analysis indicated that cAgNPs with two different sizes had inhibitory effects against these bacterial cells (Fig. 4). Based on Fig. 4, it can be concluded that smaller AgNPs are more effective in killing bacteria than larger particles, probably due to the higher surface area to volume (A/V) ratio in contact with the bacterial cells (Martínez-Castañón et al. 2008; Lago et al. 2011; Zhong et al. 2013; Ivask et al. 2014). Therefore, the bactericidal killing properties of AgNPs are size dependent. Results obtained from well diffusion experiments were also in well agreement with the dilution method results. So that smaller sized AgNPs showed faster bactericidal activity. Similar results were reported by Jeong et al. (2014). They demonstrated that 10 nm AgNPs possess higher and faster cytotoxic and antimicrobial effects compared to 100 nm sized particles against Human PBMCs and Methylobacterium spp., respectively. Dasgupta et al. (2015) have also observed that microbial, and cellular toxicology of AgNPs is size dependent. They found that smaller particles have larger surface area compared to the larger particles. Interestingly, these results agree with those presented by Martínez-Castañón et al. (2008); Lago et al. (2011); Zhong et al. (2013); Ivask et al. (2014) and Agnihotri et al. (2014). However, it is not wisely only to compare antibacterial efficacy of AgNPs on the basis of size in different studies, since it will depend on various factors, such as shape, surface chemistry, crystallinity, capping agent, dose of AgNPs, bacterial strains and composition of culture media(Hosseinpour Delavar 2014; Nafisi Bahabadi et al. 2016). So mechanism of action of AgNPs on microbes is only partially understood. There are several antimicrobial mechanisms reported by researchers (Morones et al. 2005; Kim et al. 2007; Sanpui et al. 2008; Rai et al. 2009; Chudobova et al. 2013). Overall, combinations of mechanisms include: cell membrane disruption, denaturation of ribosomes, enzymes and proteins inactivation, depletion of intracellular ATP levels, decrease DNA replication (Feng et al. 2000; Li et al. 2010; Chudobova et al. 2013), formation of free radicals (hydroxyl and ROS), oxidative stress and inactivating the respiratory chain (Chudobova et al. 2013; Zhang et al. 2014; Franci et al. 2015). This reveals that more studied are required to evaluate antibacterial mechanisms of AgNPs on bacterial pathogens in aquatics. Risk assessment studies of AgNPs in aquatic animals should also be performed before use of this technology in the aquaculture.
Conclusions
This study reveals that size of AgNPs plays a key role in the antibacterial effects. Therefore, smaller particles (16.62 nm) showed faster antimicrobial activity than larger AgNPs (22.22 nm) against luminescent V. harveyi bacteria. Finally, the results of this study suggest that AgNPs have great potential as antibacterial agents to decrease bacterial colonization and to overcome the problem of antibiotic-resistant bacteria in aquaculture.
Abbreviations
- AgNP:
-
Silver nanoparticle
- cAgNPs:
-
Colloidal silver nanoparticles
- CFU:
-
Colony-forming unit
- DLS:
-
Dynamic light scattering
- ICP:
-
Inductively coupled plasma mass spectroscopy
- MBC:
-
Minimum bactericidal concentration
- MHA:
-
Mueller-Hinton agar
- MHB:
-
Mueller-Hinton broth
- MIC:
-
Minimum inhibition concentration
- NB:
-
Nutrient broth
- OD:
-
Optical density
- PDI:
-
Polydispersity indice
- ROS:
-
Reactive oxygen species
- SPR:
-
Surface plasmon resonance
- TSA:
-
Tryptic soy agar
- TSB:
-
Tryptic soy broth
- XRD:
-
X-ray diffractometer
- ZOI:
-
Zone of inhibition
References
Agnihotri S, Mukherji S, Mukherji S (2014) Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv 4:3974–3983. doi:10.1039/C3RA44507K
Ahmad I, Beg AZ (2001) Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J Ethnopharmacol 74(4):113–123. doi:10.1016/S0378-8741(00)00335-4
Binaeian E, Rashidi AM, Attar H (2012) Toxicity study of two different synthesized silver nanoparticles on bacteria Vibrio Fischeri. Int Sch Sci Res Innov 6(7):508–514
Chatterjee S, Bandyopadhyay A, Sarkar K (2011) Effect of iron oxide and gold nanoparticles on bacterial growth leading towards biological application. J Nanobiotechnol 9(34):1–7. doi:10.1186/1477-3155-9-34
Cho KH, Park JE, Osaka T, Park SG (2005) The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim Acta 51:956–960. doi:10.1016/j.electacta.2005.04.071
Chudobova D, Maskova D, Nejdl L, Kopel P, Merlos Rodrigo MA, Adam V, Kizek R (2013) The effect of silver ions and silver nanoparticles on Staphylococcus aureus. In: Microbial pathogens and strategies for combating them: science, technology and education, pp 728–735
Dasgupta N, Ranjan S, Rajendran B, Manickam V, Ramalingam C, Avadhani S, Kumar A (2015) Antimicrobial efficacy of green synthesized drug blended silver nanoparticles against dental caries and periodontal disease causing microorganisms. Environ Sci Pollut Res 10(4):1–17
Dibrov P, Dzioba J, Gosink KK, Häse CC (2002) Chemiosmotic mechanism of antimicrobial activity of Ag+ in Vibrio cholerae. Antimicrob Agents Chemother 46:2668–2670. doi:10.1128/AAC.46.8.2668-2670.2002
Domrongpokkaphan V, Wanchaitanawong P (2006) In vitro antimicrobial activity of Bacillus spp. against Pathogenic Vibrio spp. in black tiger shrimp (Penaeus monodon). Kasetsart J (Nat Sci) 40:949–957
Emmanuel R, Palanisamy S, Chen S-M, Chelladurai K, Padmavathy S, Saravanan M, Prakash P, Ali MA, Fahad MA, Hemaid A (2015) Antimicrobial efficacy of green synthesized drug blended silver nanoparticles against dental caries and periodontal disease causing microorganisms. Mater Sci Eng, C 56(1):374–379. doi:10.1016/j.msec.2015.06.033
Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO (2000) A mechanistic study of the antibacterial effect of silver ions on E. coli and Staphylococcus aureus. J Biomed Mater Res 52:662–668. doi:10.1002/1097-4636(20001215)52:43.0.CO;2-3
Franci G, Falanga A, Galdiero S, Palomba L, Rai M, Morelli G, Galdiero M (2015) Silver nanoparticles as potential antibacterial agents. Molecules 20(5):8856–8874. doi:10.3390/molecules20058856
Hosseinpour Delavar F (2014) The effect of filters containing silver nanoparticles on the bacterial load of water used to proliferation the western white shrimp (Litopenaeus vannamei) to control contamination by Vibrio harveyi. A thesis submitted in partial fulfillment for the degree of Master of Science, Persian Gulf University of Boushehr, Iran
Ivask A, Kurvet I, Kasemets K, Blinova I, Aruoja V, Suppi S, Vija H, Käkinen K, Titma T, Heinlaan M, Visnapuu M, Koller D, Kisand V, Kahru A (2014) Size-dependent toxicity of silver nanoparticles to bacteria, yeast, algae, crustaceans and mammalian cells in vitro. PLoS One 9(7):1–14. doi:10.1371/journal.pone.0102108
Jeong Y, Lim DW, Choi J (2014) Assessment of size-dependent antimicrobial and cytotoxic properties of silver nanoparticles. Adv Mater Sci Eng. doi:10.1155/2014/763807
Johari SA, Kalbassi MR, Soltani M, Yu IJ (2015) Study of fungicidal properties of colloidal silver nanoparticles (AgNPs) on trout egg pathogen, Saprolegnia sp. Int J Aquat Biol 3(3):191–198
Khamesipour F, Noshadi E, Moradi M, Raissy M (2014) Detection of Vibrio spp. in shrimp from aquaculture sites in Iran using polymerase chain reaction (PCR). AACL Bioflux 7(1):1–7
Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY, KimYK Lee YS, Jeong DH, Cho MH (2007) Antimicrobial effects of silver nanoparticles. J Nanomed 3(1):95–101. doi:10.1016/j.nano.2014.04.007
Lago VD, de Oliveira LF, de Almeida Gonçalves K, Kobargb J, Cardoso MB (2011) Size-selective silver nanoparticles: future of biomedical devices with enhanced bactericidal properties. J Mater Chem 21(23):12267–12273. doi:10.1039/C1JM12297E
Li W-R, Xie X-B, Shi Q-S, Zeng H-Y, Ou-Yang Y-S, Chen Y-B (2010) Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl Microbiol Biotechnol 85:1115–1122. doi:10.1007/s00253-009-2159-5
Martínez-Castañón GA, Niño-Martínez N, Martínez-Gutierrez F, Martínez-Mendoza JR, Facundo R (2008) Synthesis and antibacterial activity of silver nanoparticles with different sizes. J Nanopart Res 10(6):1343–1348. doi:10.1007/s11051-008-9428-6
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Tapia Ramírez J, Yacaman MJ (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16:2346–2353. doi:10.13005/ojc/300341
Nafisi Bahabadi M, Hosseinpour Delavar F, Mirbaksh M, Niknam KH, Johari SA (2016) Assessing antibacterial effect of filter media coated with silver nanoparticles against Bacillus spp. Iran South Med J 19(1):1–14
Petrus EM, Tinakumari S, Chai LC, Ubong A, Tunung R, Elexson N, Chai LF, Son R (2011) A study on the minimum inhibitory concentration and minimum bactericidal concentration of nano colloidal silver on food-borne pathogens. Int Food Res J 18:55–66
Rahman Nia J (2011) Preparation of colloidal nanosilver. Google US Patent 2011
Rai M, Yadav A, Gade A (2009) Silver nanoparticles: as a new generation of antimicrobials. Biotechnol Adv 27:76–83. doi:10.1016/j.biotechadv.2008.09.002
Ramesh K, Natarajan M, Sridhar H, Umamaheswari S (2014) Virulence determination among Vibrio harveyi hatchery isolates through haemolysis and growth constraint. Glob J Bio-Sci Biotechnol 3(1):109–114
Sanpui P, Murugadoss A, Prasad PVD, Ghos SS, Chattopadhyay A (2008) The antibacterial properties of a novel chitosan–Ag-nanoparticle composite. Int J Food Microbiol 124:142–146. doi:10.1016/j.ijfoodmicro
Sarkar B, Mahanty A, Netam SP, Mishra S, Pradhan N, Samanta M (2012) Inhibitory role of silver nanoparticles against important fish pathogen, Aeromonas hydrophila. Int J Nanomater Biostruct 2(4):70–74
Savithramma N, Linga Rao M, Rukmini K, Suvarnalatha Devi P (2011) Antimicrobial activity of silver nanoparticles synthesized by using medicinal plants. Int J ChemTech Res 3(3):1394–1402
Soltani M, Ghodratnema M, Ahari H, Ebrahimzadeh Musav HA, Atee M, Dastmachi F, Rahmanya J (2009) The inhibitory effect of silver nanoparticles on the bacterial fish pathogens, Streptococcus iniae, Lactococcus garvieae, Yersinia ruckeri and Aeromonas hydrophila. Int J Vet Res 3(2):137–142
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci 275:177–182. doi:10.1016/j.jcis.2004.02.01
Vaseeharan B, Ramasamy P, Chen JC (2010) Antibacterial activity of silver nanoparticles (AgNPs) synthesized by tea leaf extracts against pathogenic Vibrio harveyi and its protective efficacy on juvenile Feneropenaeus indicus. Lett Appl Microbiol 50:352–356. doi:10.1111/j.1472-765X.2010.02799.x
Vaseeharan B, Sargunar CG, Lin YC, Chen GC (2012) Green synthesis of silver nanoparticles through Calotropis gigantea leaf extracts and evaluation of antibacterial activity against Vibrio alginolyticus. Nanotechnol Dev 2(3):12–16. doi:10.4081/nd.2012.e3
Zhang T, Wang L, Chen Q, Chen C (2014) Cytotoxic potential of silver nanoparticles. Yonsei Med J 55(2):283–291. doi:10.3349/ymj.2014.55.2.283
Zhong L, Kaifeng R, Ju L, Hao Y, Rong C (2013) Size-dependent antibacterial activities of silver nanoparticles against oral anaerobic pathogenic bacteria. J Mater Sci Mater Med 24:1465–1471. doi:10.1007/s10856-013-4894-5
Acknowledgments
This study was financially supported by Persian Gulf University of Bushehr, Iran and Iranian Shrimp Research Institute. The authors are thankful to Dr. Mohammad Hosseinpour Delavar and Ms. Esmat Mohammadi for their helpful assistance in antimicrobial tests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nafisi Bahabadi, M., Hosseinpour Delavar, F., Mirbakhsh, M. et al. Assessment of antibacterial activity of two different sizes of colloidal silver nanoparticle (cAgNPs) against Vibrio harveyi isolated from shrimp Litopenaeus vannamei . Aquacult Int 25, 463–472 (2017). https://doi.org/10.1007/s10499-016-0043-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-016-0043-8