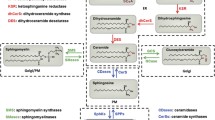
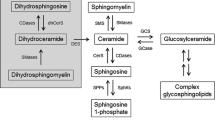
Abstract
Glycosphingolipids (GSLs) are a family of bioactive lipids that in addition to their role in the regulation of structural properties of membrane bilayers have emerged as crucial players in many biological processes and signal transduction pathways. Rather than being uniformly distributed within membrane bilayers, GSLs are localized in selective domains called lipid rafts where many signaling platforms operate. One of the most important functions of GSLs, particularly ceramide, is their ability to regulate cell death pathways and hence cell fate. This complex role is accomplished by the ability of GSLs to act in distinct subcellular strategic centers, such as mitochondria, endoplasmic reticulum (ER) or lysosomes to mediate apoptosis, ER stress, autophagy, lysosomal membrane permeabilization and necroptosis. Hence better understanding the role of GSLs in cell death may be of relevance for a number of pathological processes and diseases, including neurodegeneration, metabolic liver diseases and cancer.


Similar content being viewed by others
References
Hannun YA, Obeid LM (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9:139–150
Ogretmen B, Hannun YA (2004) Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer 4:604–616
Chavez JA, Summers SA (2012) A ceramide-centric view of insulin resistance. Cell Metab 15:585–594
Maceyka M, Spiegel S (2014) Sphingolipid metabolites in inflammatory disease. Nature 510:58–67
Morales A, Lee H, Goñi F, Kolesnick R, Fernandez-Checa J (2007) Sphingolipids and cell death. Apoptosis 12:923–939
Mizutani Y, Kihara A, Chiba H, Tojo H, Igarashi Y (2008) 2-Hydroxy-ceramide synthesis by ceramide synthase family: enzymatic basis for the preference of FA chain length. J Lipid Res 49:2356–2364
Park JW, Park WJ, Futerman AH (1841) Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochim Biophys Acta 671–681:2014
Grösch S, Schiffmann S, Geisslinger G (2012) Chain length-specific properties of ceramides. Prog Lipid Res 51:50–62
Pewzner-Jung Y, Brenner O, Braun S, Laviad EL, Ben-Dor S, Feldmesser E, Horn-Saban S, Amann-Zalcenstein D, Raanan C, Berkutzki T, Erez-Roman R, Ben-David O, Levy M, Holzman D, Park H, Nyska A, Merrill AH Jr, Futerman AH (2010) A critical role for ceramide synthase 2 in liver homeostasis: II. Insights into molecular changes leading to hepatopathy. J Biol Chem 285:10911–10923
Turpin SM, Nicholls HT, Willmes DM, Mourier A, Brodesser S, Wunderlich CM, Mauer J, Xu E, Hammerschmidt P, Brönneke HS, Trifunovic A, LoSasso G, Wunderlich FT, Kornfeld JW, Blüher M, Krönke M, Brüning JC (2014) Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab 20:678–686
Raichur S, Wang ST, Chan PW, Li Y, Ching J, Chaurasia B, Dogra S, Öhman MK, Takeda K, Sugii S, Pewzner-Jung Y, Futerman AH, Summers SA (2014) CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab 20:687–695
Bartke N, Hannun YA (2009) Bioactive sphingolipids: metabolism and function. J Lipid Res 50:S91–S96
Breslow DK, Weissman JS (2010) Membranes in balance: mechanisms of sphingolipid homeostasis. Mol Cell 40:267–279
Bikman BT, Summers SA (2011) Ceramides as modulators of cellular and whole-body metabolism. J Clin Invest 121:4222–4230
Holland WL, Summers SA (2008) Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev 29:381–402
Paumen MB, Ishida Y, Muramatsu M, Yamamoto M, Honjo T (1997) Inhibition of carnitine palmitoyltransferase i augments sphingolipid synthesis and palmitate-induced apoptosis. J Biol Chem 272:3324–3329
Straczkowski M, Kowalska I, Baranowski M, Nikolajuk A, Otziomek E, Zabielski P, Adamska A, Blachnio A, Gorski J, Gorska M (2007) Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia 50:2366–2373
Watt MJ, Barnett AC, Bruce CR, Schenk S, Horowitz JF, Hoy AJ (2012) Regulation of plasma ceramide levels with fatty acid oversupply: evidence that the liver detects and secretes de novo synthesised ceramide. Diabetologia 55:2741–2746
Breslow DK, Collins SR, Bodenmiller B, Aebersold R, Simons K, Shevchenko A, Ejsing CS, Weissman JS (2010) Orm family proteins mediate sphingolipid homeostasis. Nature 463:1048–1053
Roelants FM, Breslow DK, Muir A, Weissman JS, Thorner J (2011) Protein kinase Ypk1 phosphorylates regulatory proteins Orm1 and Orm2 to control sphingolipid homeostasis in Saccharomyces cerevisiae. Proc Natl Acad Sci 108:19222–19227
Aronova S, Wedaman K, Aronov PA, Fontes K, Ramos K, Hammock BD, Powers T (2008) Regulation of ceramide biosynthesis by TOR complex 2. Cell Metab 7:148–158
Dickson RC (2008) Thematic review series: sphingolipids. New insights into sphingolipid metabolism and function in budding yeast. J Lipid Res 49:909–921
Berchtold D, Piccolis M, Chiaruttini N, Riezman I, Riezman H, Roux A, Walther TC, Loewith R (2012) Plasma membrane stress induces relocalization of Slm proteins and activation of TORC2 to promote sphingolipid synthesis. Nat Cell Biol 14:542–547
Siow DL, Wattenberg BW (2012) Mammalian ORMDL proteins mediate the feedback response in ceramide biosynthesis. J Biol Chem 287:40198–40204
Gupta SD, Gable K, Alexaki A, Chandris P, Proia RL, Dunn TM, Harmon JM (2014) Expression of the ORMDLS, modulators of serine palmitoyltransferase, is regulated by sphingolipids in mammalian cells. J Biol Chem. doi:10.1074/jbc.M114.588236
Canals D, Perry DM, Jenkins RW, Hannun YA (2011) Drug targeting of sphingolipid metabolism: sphingomyelinases and ceramidases. Br J Pharmacol 163:694–712
Smith EL, Schuchman EH (2008) The unexpected role of acid sphingomyelinase in cell death and the pathophysiology of common diseases. FASEB J 22:3419–3431
Angulo S, Morales A, Danese S, Llacuna L, Masamunt MC, Pultz N, Cifone MG, De Simone C, Delgado S, Vila J, Panes J, Donskey C, Fernandez-Checa JC, Fiocchi C, Sans M (2011) Probiotic sonicates selectively induce mucosal immune cells apoptosis through ceramide generation via neutral sphingomyelinase. PLoS One 6:e16953
Coll O, Morales A, Fernández-Checa JC, Garcia-Ruiz C (2007) Neutral sphingomyelinase-induced ceramide triggers germinal vesicle breakdown and oxidant-dependent apoptosis in Xenopus laevis oocytes. J Lipid Res 48:1924–1935
Marí M, Fernández-Checa JC (2007) Sphingolipid signalling and liver diseases. Liver Int 27:440–450
Garcia-Ruiz C, Colell A, Mari M, Morales A, Calvo M, Enrich C, Fernandez-Checa JC (2003) Defective TNF-α-mediated hepatocellular apoptosis and liver damage in acidic sphingomyelinase knockout mice. J Clin Invest 111:197–208
Mari M, Colell A, Morales A, Paneda C, Varela-Nieto I, Garcia-Ruiz C, Fernandez-Checa JC (2004) Acidic sphingomyelinase downregulates the liver-specific methionine adenosyltransferase 1A, contributing to tumor necrosis factor-induced lethal hepatitis. J Clin Invest 113:895–904
Lin T, Genestier L, Pinkoski MJ, Castro A, Nicholas S, Mogil R, Paris F, Fuks Z, Schuchman EH, Kolesnick RN, Green DR (2000) Role of acidic sphingomyelinase in Fas/CD95-mediated cell death. J Biol Chem 275:8657–8663
Lang PA, Schenck M, Nicolay JP, Becker JU, Kempe DS, Lupescu A, Koka S, Eisele K, Klarl BA, Rubben H, Schmid KW, Mann K, Hildenbrand S, Hefter H, Huber SM, Wieder T, Erhardt A, Haussinger D, Gulbins E, Lang F (2007) Liver cell death and anemia in Wilson disease involve acid sphingomyelinase and ceramide. Nat Med 13:164–170
Fucho R, Martinez L, Baulies A, Torres S, Tarrats N, Fernandez A, Ribas V, Astudillo AM, Balsinde J, Garcia-Roves P, Elena M, Bergheim I, Lotersztajn S, Trautwein C, Appelqvist H, Paton AW, Paton JC, Czaja MJ, Kaplowitz N, Fernandez-Checa JC, Garcia-Ruiz C (2014) Asmase regulates autophagy and lysosomal membrane permeabilization and its inhibition prevents early stage nonalcoholic steatohepatitis. J Hepatol. doi:10.1016/j.jhep.2014.06.009
Fernandez A, Matias N, Fucho R, Ribas V, Von Montfort C, Nuño N, Baulies A, Martinez L, Tarrats N, Mari M, Colell A, Morales A, Dubuquoy L, Mathurin P, Bataller R, Caballeria J, Elena M, Balsinde J, Kaplowitz N, Garcia-Ruiz C, Fernandez-Checa JC (2013) ASMase is required for chronic alcohol induced hepatic endoplasmic reticulum stress and mitochondrial cholesterol loading. J Hepatol 59:805–813
Jenkins RW, Idkowiak-Baldys J, Simbari F, Canals D, Roddy P, Riner CD, Clarke CJ, Hannun YA (2011) A novel mechanism of lysosomal acid sphingomyelinase maturation: requirement for carboxyl-terminal proteolytic processing. J Biol Chem 286:3777–3788
Paris F, Grassmé H, Cremesti A, Zager J, Fong Y, Haimovitz-Friedman A, Fuks Z, Gulbins E, Kolesnick R (2001) Natural ceramide reverses fas resistance of acid sphingomyelinase(−/−) hepatocytes. J Biol Chem 276:8297–8305
Llacuna L, Mari M, Garcia-Ruiz C, Fernandez-Checa JC, Morales A (2006) Critical role of acidic sphingomyelinase in murine hepatic ischemia-reperfusion injury. Hepatology (Hoboken, NJ, U. S.) 44:561–572
Grassme H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, Kolesnick R, Gulbins E (2001) CD95 signaling via ceramide-rich membrane rafts. J Biol Chem 276:20589–20596
Cremesti A, Paris F, Grassmé H, Holler N, Tschopp J, Fuks Z, Gulbins E, Kolesnick R (2001) Ceramide enables fas to cap and kill. J Biol Chem 276:23954–23961
Grassmé H, Cremesti A, Kolesnick R, Gulbins E (2003) Ceramide-mediated clustering is required for CD95-DISC formation. Oncogene 22:5457–5470
Perrotta C, Bizzozero L, Cazzato D, Morlacchi S, Assi E, Simbari F, Zhang Y, Gulbins E, Bassi MT, Rosa P, Clementi E (2010) Syntaxin 4 is required for acid sphingomyelinase activity and apoptotic function. J Biol Chem 285:40240–40251
Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, Saglietti L, Schuchman EH, Furlan R, Clementi E, Matteoli M, Verderio C (2009) Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J 28:1043–1054
Ridgway ND (2000) Interactions between metabolism and intracellular distribution of cholesterol and sphingomyelin. Biochimica et Biophysica Acta (BBA) - Mol Cell Biol Lipids 1484:129–141
Slotte JP (1999) Sphingomyelin–cholesterol interactions in biological and model membranes. Chem Phys Lipids 102:13–27
Li X, Xu M, Pitzer A, Xia M, Boini K, Li P-L, Zhang Y (2014) Control of autophagy maturation by acid sphingomyelinase in mouse coronary arterial smooth muscle cells: protective role in atherosclerosis. J Mol Med 92:473–485
Alayoubi AM, Wang JC, Au BC, Carpentier S, Garcia V, Dworski S, El-Ghamrasni S, Kirouac KN, Exertier MJ, Xiong ZJ, Privé GG, Simonaro CM, Casas J, Fabrias G, Schuchman EH, Turner PV, Hakem R, Levade T, Medin JA (2013) Systemic ceramide accumulation leads to severe and varied pathological consequences. EMBO Mol Med 5:827–842
Chipuk JE, Mcstay GP, Bharti A, Kuwana T, Clarke CJ, Siskind LJ, Obeid LM, Green (2012) Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell 148:988–1000
Lee SH, Seo GS, Park P-H, Choi J-Y, Park YN, Kim HK, Chae K-S, Sohn DH (2003) Increased expression of O-acetyl disialoganglioside synthase during rat liver fibrogenesis relates to stellate cell activation. Biochem Biophys Res Commun 303:954–961
Huitema K, Van Den Dikkenberg J, Brouwers JFHM, Holthuis JCM (2004) Identification of a family of animal sphingomyelin synthases. EMBO J 23:33–44
Vacaru AM, Tafesse FG, Ternes P, Kondylis V, Hermansson M, Brouwers JFHM, Somerharju P, Rabouille C, Holthuis JCM (2009) Sphingomyelin synthase-related protein SMSr controls ceramide homeostasis in the ER. J Cell Biol 185:1013–1027
D’angelo G, Polishchuk E, Tullio GD, Santoro M, Campli AD, Godi A, West G, Bielawski J, Chuang C-C, Van Der Spoel AC, Platt FM, Hannun YA, Polishchuk R, Mattjus P, De Matteis MA (2007) Glycosphingolipid synthesis requires FAPP2 transfer of glucosylceramide. Nature 449:62–67
Mao C, Obeid LM (2008) Ceramidases: regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochimica et Biophysica Acta (BBA) - Mol Cell Biol Lipids 1781:424–434
Hassler DF, Bell RM (1993) Ceramidases: enzymology and metabolic roles. Adv Lipid Res 26:49–57
Franzen R, Pautz A, Bräutigam L, Geisslinger G, Pfeilschifter J, Huwiler A (2001) Interleukin-1β induces chronic activation and de novo synthesis of neutral ceramidase in renal mesangial cells. J Biol Chem 276:35382–35389
Ramirez De Molina A, De La Cueva A, Machado-Pinilla R, Rodriguez-Fanjul V, Gomez Del Pulgar T, Cebrian A, Perona R, Lacal JC (2012) Acid ceramidase as a chemotherapeutic target to overcome resistance to the antitumoral effect of choline kinase α inhibition. Curr Cancer Drug Targets 12:617–624
Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind JS, Spiegel S (1996) Suppression of ceramide-mediated programmed cell death. Nature 381:800–803
Pyne NJ, Pyne S (2010) Sphingosine 1-phosphate and cancer. Nat Rev Cancer 10:489–503
Perez GI, Knudson CM, Leykin L, Korsmeyer SJ, Tilly JL (1997) Apoptosis-associated signaling pathways are required for chemotherapy-mediated female germ cell destruction. Nat Med 3:1228–1232
Visentin B, Vekich JA, Sibbald BJ, Cavalli AL, Moreno KM, Matteo RG, Garland WA, Lu Y, Yu S, Hall HS, Kundra V, Mills GB, Sabbadini RA (2006) Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell 9:225–238
Cingolani F, Casasampere M, Sanllehi P, Casas J, Bujons J, Fabrias G (2014) Inhibition of dihydroceramide desaturase activity by the sphingosine kinase inhibitor SKI II. J Lipid Res 55:1711–1720
Van Veldhoven PP (2000) Sphingosine-1-phosphate lyase. Methods Enzymol 311:244–254
Le Stunff H, Giussani P, Maceyka M, Lépine S, Milstien S, Spiegel S (2007) Recycling of sphingosine is regulated by the concerted actions of sphingosine-1-phosphate phosphohydrolase 1 and sphingosine kinase 2. J Biol Chem 282:34372–34380
Liu H, Sugiura M, Nava VE, Edsall LC, Kono K, Poulton S, Milstien S, Kohama T, Spiegel S (2000) Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem 275:19513–19520
Spiegel S, Milstien S (2003) Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol 4:397–407
Taylor RC, Cullen SP, Martin SJ (2008) Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 9:231–241
García-Ruiz C, Colell A, Marí M, Morales A, Fernández-Checa JC (1997) Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species: role of mitochondrial glutathione. J Biol Chem 272:11369–11377
Gudz TI, Tserng K-Y, Hoppel CL (1997) direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J Biol Chem 272:24154–24158
Dai Q, Liu J, Chen J, Durrant D, Mcintyre TM, Lee RM (2004) Mitochondrial ceramide increases in UV-irradiated HeLa cells and is mainly derived from hydrolysis of sphingomyelin. Oncogene 23:3650–3658
Birbes H, Luberto C, Hsu Y-T, El Bawab S, Hannun YA, Obeid LM (2005) A mitochondrial pool of sphingomyelin is involved in TNFalpha-induced Bax translocation to mitochondria. Biochem J 386:445–451
El Bawab S, Roddy P, Qian T, Bielawska A, Lemasters JJ, Hannun YA (2000) Molecular cloning and characterization of a human mitochondrial ceramidase. J Biol Chem 275:21508–21513
Bionda C, Portoukalian J, Schmitt D, Rodriguez-Lafrasse C, Ardail D (2004) Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? Biochem J 382:527–533
De Maria R, Rippo MR, Schuchman EH, Testi R (1998) Acidic sphingomyelinase (ASM) is necessary for Fas-induced GD3 ganglioside-accumulation and efficient apoptosis of lymphoid cells. J Exp Med 187:897–902
García-Ruiz C, Colell A, París R, Fernández-Checa JC (2000) Direct interaction of GD3 ganglioside with mitochondria generates reactive oxygen species followed by mitochondrial permeability transition, cytochrome c release, and caspase activation. FASEB J 14:847–858
Kristal BS, Brown AM (1999) Apoptogenic ganglioside GD3 directly induces the mitochondrial permeability transition. J Biol Chem 274:23169–23175
Rippo MR, Malisan F, Ravagnan L, Tomassini B, Condo I, Costantini P, Susin SA, Rufini A, Todaro M, Kroemer G, Testi R (2000) GD3 ganglioside directly targets mitochondria in a bcl-2-controlled fashion. FASEB J 14:2047–2054
García-Ruiz C, Colell A, Morales A, Calvo MA, Enrich C, Fernández-Checa JC (2002) Trafficking of ganglioside GD3 to mitochondria by tumor necrosis factor-α. J Biol Chem 277:36443–36448
Brenner C, Kniep B, Maillier E, Martel C, Franke C, Röber N, Bachmann M, Rieber EP, Sandhoff R (2010) GD3–7-aldehyde is an apoptosis inducer and interacts with adenine nucleotide translocase. Biochem Biophys Res Commun 391:248–253
Sorice M, Mattei V, Matarrese P, Garofalo T, Tinari A, Gambardella L, Ciarlo L, Manganelli V, Tasciotti V, Misasi R, Malorni W (2012) Dynamics of mitochondrial raft-like microdomains in cell life and death. Commun Integr Biol 5:217–219
Lee H, Rotolo JA, Mesicek J, Penate-Medina T, Rimner A, Liao W-C, Yin X, Ragupathi G, Ehleiter D, Gulbins E, Zhai D, Reed JC, Haimovitz-Friedman A, Fuks Z, Kolesnick R (2011) Mitochondrial ceramide-rich macrodomains functionalize Bax upon irradiation. PLoS One 6:e19783
Ciarlo L, Manganelli V, Garofalo T, Matarrese P, Tinari A, Misasi R, Malorni W, Sorice M (2010) Association of fission proteins with mitochondrial raft-like domains. Cell Death Differ 17:1047–1058
Galluzzi L, Bravo-San Pedro JM, Kroemer G (2014) Organelle-specific initiation of cell death. Nat Cell Biol 16:728–736
Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11:619–633
Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC (2003) Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene 22:8608–8618
Lloyd-Evans E, Pelled D, Riebeling C, Bodennec J, De-Morgan A, Waller H, Schiffmann R, Futerman AH (2003) Glucosylceramide and glucosylsphingosine modulate calcium mobilization from brain microsomes via different mechanisms. J Biol Chem 278:23594–23599
Pelled D, Lloyd-Evans E, Riebeling C, Jeyakumar M, Platt FM, Futerman AH (2003) Inhibition of calcium uptake via the sarco/endoplasmic reticulum Ca2+-atpase in a mouse model of sandhoff disease and prevention by treatment with N-butyldeoxynojirimycin. J Biol Chem 278:29496–29501
Tessitore A, Del P Martin M, Sano R, Ma Y, Mann L, Ingrassia A, Laywell ED, Steindler DA, Hendershot LM, D’azzo A (2004) GM1-ganglioside-mediated activation of the unfolded protein response causes neuronal death in a neurodegenerative gangliosidosis. Mol Cell 15:753–766
Carracedo A, Lorente M, Egia A, Blázquez C, García S, Giroux V, Malicet C, Villuendas R, Gironella M, González-Feria L, Piris MÁ, Iovanna JL, Guzmán M, Velasco G (2006) The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer Cell 9:301–312
Boslem E, Weir JM, Macintosh G, Sue N, Cantley J, Meikie PJ, Biden TJ (2013) Alteration of endoplasmic reticulum lipid rafts contributes to lipotoxicity in pancreatic β-cells. J Biol Chem 288:26569–26582
Gozuacik D, Kimchi A (2007) Autophagy and cell death. Curr Top Dev Biol 78:217–245
Yu L, Wan F, Dutta S, Welsh S, Liu Z, Freundt E, Baehrecke EH, Lenardo M (2006) Autophagic programmed cell death by selective catalase degradation. Proc Natl Acad Sci USA 103:4952–4957
Li Y, Li S, Qin X, Hou W, Dong H, Yao L, Xiong L (2014) The pleiotropic roles of sphingolipid signaling in autophagy. Cell Death Dis 5:e1245
Schubert KM, Scheid MP, Duronio V (2000) Ceramide inhibits protein kinase B/Akt by promoting dephosphorylation of serine 473. J Biol Chem 275:13330–13335
Edinger AL (2009) Starvation in the midst of plenty: making sense of ceramide-induced autophagy by analyzing nutrient transporter expression. Biochem Soc Trans 37:253–258
Guenther GG, Peralta ER, Rosales KR, Wong SY, Siskind LJ, Edinger AL (2008) Ceramide starves cells to death by downregulating nutrient transporter proteins. Proc Natl Acad Sci USA. 105:17402–17407
Scarlatti F, Bauvy C, Ventruti A, Sala G, Cluzeaud F, Vandewalle A, Ghidoni R, Codogno P (2004) Ceramide-mediated Macroautophagy involves inhibition of protein kinase B and up-regulation of beclin 1. J Biol Chem 279:18384–18391
Young MM, Kester M, Wang H-G (2013) Sphingolipids: regulators of crosstalk between apoptosis and autophagy. J Lipid Res 54:5–19
Spassieva SD, Mullen TD, Townsend DM, Obeid LM (2009) Disruption of ceramide synthesis by CerS2 down-regulation leads to autophagy and the unfolded protein response. Biochem J 424:273–283
Russo SB, Baicu CF, Van Laer A, Geng T, Kasiganesan H, Zile MR, Cowart LA (2012) Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J Clin Invest 122:3919–3930
Beauchamp E, Goenaga D, Le Bloch J, Catheline D, Legrand P, Rioux V (2007) Myristic acid increases the activity of dihydroceramide Delta4-desaturase 1 through its N-terminal myristoylation. Biochimie 89:1553–1561
Jiang W, Ogretmen B (2014) Autophagy paradox and ceramide. Biochimica et Biophysica Acta (BBA) - Mol Cell Biol Lipids 1841:783–792
Park MA, Zhang G, Martin AP, Hamed H, Mitchell C, Hylemon PB, Graf M, Rahmani M, Ryan K, Liu X, Spiegel S, Norris J, Fisher PB, Grant S, Dent P (2008) Vorinostat and sorafenib increase ER stress, autophagy and apoptosis via ceramide-dependent CD95 and PERK activation. Cancer Biol Ther 7:1648–1662
Li DD, Wang LL, Deng R, Tang J, Shen Y, Guo JF, Wang Y, Xia LP, Feng GK, Liu QQ, Huang WL, Zeng YX, Zhu XF (2009) The pivotal role of c-Jun NH2-terminal kinase-mediated Beclin 1 expression during anticancer agents-induced autophagy in cancer cells. Oncogene 28:886–898
Zeidan YH, Jenkins RW, Hannun YA (2008) Remodeling of cellular cytoskeleton by the acid sphingomyelinase/ceramide pathway. J Cell Biol 181:335–350
Matarrese P, Garofalo T, Manganelli V, Gambardella L, Marconi M, Grasso M, Tinari A, Misasi R, Malorni W, Sorice M (2014) Evidence for the involvement of GD3 ganglioside in autophagosome formation and maturation. Autophagy 10:750–765
Hwang J, Lee S, Lee JT, Kwon TK, Kim DR, Kim H, Park HC, Suk K (2010) Gangliosides induce autophagic cell death in astrocytes. Br J Pharmacol 159:586–603
Li ZZ, Berk M, Mcintyre TM, Gores GJ, Feldstein AE (2008) The lysosomal-mitochondrial axis in free fatty acid-induced hepatic lipotoxicity. Hepatology (Hoboken, NJ, U. S.) 47:1495–1503
Petersen NHT, Olsen OD, Groth-Pedersen L, Ellegaard A-M, Bilgin M, Redmer S, Ostenfeld MS, Ulanet D, Dovmark TH, Lønborg A, Vindeløv SD, Hanahan D, Arenz C, Ejsing CS, Kirkegaard T, Rohde M, Nylandsted J, Jäättelä M (2013) Transformation-associated changes in sphingolipid metabolism sensitize cells to lysosomal cell death induced by inhibitors of acid sphingomyelinase. Cancer Cell 24:379–393
Appelqvist H, Nilsson C, Garner B, Brown AJ, Kågedal K, Öllinger K (2011) Attenuation of the lysosomal death pathway by lysosomal cholesterol accumulation. Am J Pathol 178:629–639
Ullio C, Casas J, Brunk UT, Sala G, Fabriàs G, Ghidoni R, Bonelli G, Baccino FM, Autelli R (2012) Sphingosine mediates TNFα-induced lysosomal membrane permeabilization and ensuing programmed cell death in hepatoma cells. J Lipid Res 53:1134–1143
Villamil Giraldo AM, Appelqvist H, Ederth T, Öllinger K (2014) Lysosomotropic agents: impact on lysosomal membrane permeabilization and cell death. Biochem Soc Trans 42:1460–1464
Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D (1995) Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature 376:167–170
Doi TS, Takahshi T, Taguchi O, Azuma T, Obata Y (1997) NF-kappa B RelA-deficient lymphocytes: normal development of T cells and B cells, impaired production of IgA and IgG1 and reduced proliferative responses. J Exp Med 185:953–961
Schütze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Krönke M (1992) TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced “acidic” sphingomyelin breakdown. Cell 71:765–776
Dbaibo GS, Obeid LM, Hannun YA (1993) Tumor necrosis factor-alpha (TNF-alpha) signal transduction through ceramide. Dissociation of growth inhibitory effects of TNF-alpha from activation of nuclear factor-kappa B. J Biol Chem 268:17762–17766
Betts JC, Agranoff AB, Nabel GJ, Shayman JA (1994) Dissociation of endogenous cellular ceramide from NF-kappa B activation. J Biol Chem 269:8455–8458
Colell A, García-Ruiz C, Roman J, Ballesta A, Fernández-Checa JC (2001) Ganglioside GD3 enhances apoptosis by suppressing the nuclear factor-kB-dependent survival pathway. FASEB J 15:1068–1070
Paris R, Morales A, Coll O, Sánchez-Reyes A, García-Ruiz C, Fernández-Checa JC (2002) Ganglioside GD3 sensitizes human hepatoma cells to cancer therapy. J Biol Chem 277:49870–49876
Malisan F, Franchi L, Tomassini B, Ventura N, Condo I, Rippo MR, Rufini A, Liberati L, Nachtigall C, Kniep B, Testi R (2002) Acetylation suppresses the proapoptotic activity of GD3 ganglioside. J Exp Med 196:1535–1541
Mukherjee K, Chava AK, Mandal C, Dey SN, Kniep B, Chandra S, Mandal C (2008) O-acetylation of GD3 prevents its apoptotic effect and promotes survival of lymphoblasts in childhood acute lymphoblastic leukaemia. J Cell Biochem 105:724–734
Birks SM, Danquah JO, King L, Vlasak R, Gorecki DC, Pilkington GJ (2011) Targeting the GD3 acetylation pathway selectively induces apoptosis in glioblastoma. Neuro-Oncology 13:950–960
Cazet A, Groux-Degroote S, Teylaert B, Kwon K-M, Lehoux S, Slomianny C, Kim C-H, Le Bourhis X, Delannoy P (2009) GD3 synthase overexpression enhances proliferation and migration of MDA-MB-231 breast cancer cells. Biol Chem 390:601–609
Lluis JM, Llacuna L, Von MC, Barcena C, Enrich C, Morales A, Fernandez-Checa JC (2009) GD3 synthase overexpression sensitizes hepatocarcinoma cells to hypoxia and reduces tumor growth by suppressing the cSrc/NF-kappaB survival pathway. PLoS ONE 4:e8059
Chen HY, Challa AK, Varki A (2006) 9-O-Acetylation of exogenously added ganglioside GD3: the GD3 molecule induces its own O-acetylation machinery. J Biol Chem 281:7825–7833
Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G (2010) Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 11:700–714
Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J (2009) RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325:332–336
Platt FM (2014) Sphingolipids lysosomal storage disorders. Nature 510:68–75
Vitner EB, Salomon R, Farfel-Becker T, Meshcheriakova A, Ali M, Klein AD, Platt FM, Cox TM, Futerman AH (2014) RIPK3 as a potential therapeutic target for Gaucher’s disease. Nat Med 20:204–208
Ryland LK, Fox TE, Liu X, Loughran TP, Kester M (2011) Dysregulation of sphingolipid metabolism in cancer. Cancer Biol Ther 11:138–149
Tagaram HRS, Divittore NA, Barth BM, Kaiser JM, Avella D, Kimchi ET, Jiang Y, Isom HC, Kester M, Staveley-O’carroll KF (2011) Nanoliposomal ceramide prevents in vivo growth of hepatocellular carcinoma. Gut 60:695–701
Garcia-Ruiz C, Mato JM, Vance D, Kaplowitz N, Fernández-Checa JC (2014) Acid sphingomyelinase-ceramide system in steatohepatitis: a novel target regulating multiple pathways. J Hepatol 62:219–233
Grimm MO, Zimmwe VC, Lehmann J, Grimm H, Hartmann T (2013) The impact of cholesterol, DHA, and sphingolipids on Alzheimer’s disease. Biomed Res Int 2013:814390
Lee JK, Jin HK, Park MH, Kim BR, Lee PH, Nakauchi H, Carter JE, He X, Schuchman EH, Bae JS (2014) Acid sphingomyelinase modulates the autophagic process by controlling lysosomal biogenesis in Alzheimer’s disease. J Exp Med 211:1551–1570
Acknowledgments
The work was supported by Grants SAF-2011-23031, SAF-2012-34831 from Plan Nacional de I+D, Spain, the center Grant P50-AA-11999 Research Center for Liver and Pancretic Diseases funded by NIAAA/NIH, a Grant from Fundació Marató de TV3, La Mutua Madrileña, PI11/0325 (META) Grant from the Instituto Salud Carlos III, and by the support of CIBEREHD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garcia-Ruiz, C., Morales, A. & Fernández-Checa, J.C. Glycosphingolipids and cell death: one aim, many ways. Apoptosis 20, 607–620 (2015). https://doi.org/10.1007/s10495-015-1092-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-015-1092-6