Abstract
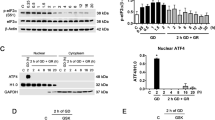
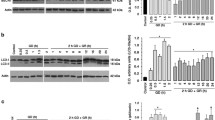
Glucose is the main energy source in brain and it is critical for correct brain functioning. Type 1 diabetic patients might suffer from severe hypoglycemia if exceeding insulin administration, which can lead to acute brain injury if not opportunely corrected. The mechanisms leading to hypoglycemic brain damage are not completely understood and the role of endoplasmic reticulum (ER) stress has not been studied. ER stress resulting from the accumulation of unfolded or misfolded proteins in the ER is counteracted by the unfolded protein response (UPR). When the UPR is sustained, apoptotic death might take place. We have examined UPR activation during glucose deprivation (GD) in hippocampal cultured neurons and its role in the induction of apoptosis. Activation of the PERK pathway of the UPR was observed, as increased phosphorylation of eIF2α and elevated levels of the transcription factor ATF4, occurred 30 min after GD and the levels of the chaperone protein, GRP78 and the transcription factor CHOP, increased after 2 h of GD. In addition, we observed an early activation of caspase-7 and 12 during GD, while caspase-3 activity increased only transiently during glucose reintroduction. Inhibition of caspase-3/7 and the calcium-dependent protease, calpain, significantly decreased caspase-12 activity. The ER stress inhibitor, salubrinal prevented neuronal death and caspase-12 activity. Results suggest that the PERK pathway of the UPR is involved in GD-induced apoptotic neuronal death through the activation of caspase-12, rather than the mitochondrial-dependent caspase pathway. In addition, we show that calpain and caspase-7 are soon activated after GD and mediate caspase-12 activation and neuronal death.





Similar content being viewed by others
Abbreviations
- ER:
-
Endoplasmic reticulum
- UPR:
-
Unfolded protein response
- GD:
-
Glucose deprivation
- GR:
-
Glucose reintroduction,
- GRP78:
-
78-kDa Glucose-regulated protein
- eIF2α:
-
Eukaryotic translation initiation factor-2
- ATF4:
-
Activating transcription factor 4
- CHOP:
-
C/EBP homologous protein
- QVDOPH:
-
Q-Val-Asp-OPH
- DEVDCHO:
-
Acetyl-Asp-Glu-Val-Asp-aldehyde
- MDL-28170:
-
N-Benzyloxycarbonylvalylphenylalaninal
- Q-ATAD:
-
Q-Ala-Thr-Ala-Asp(OMe)-OPH
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide
- STS:
-
Staurosporine
- Tg:
-
Thapsigargin
- Tm:
-
Tunicamycin
References
Auer RN, Kalimo H, Olsson Y, Siesjö BK (1985) The temporal evolution of hypoglycemic brain damage. I Light- and electron-microscopic findings in the rat cerebral cortex. Acta Neuropathol 67:13–24
Auer RN, Kalimo H, Olsson Y, Siesjö BK (1985) The temporal evolution of hypoglycemic brain damage. II Light- and electron-microscopic findings in the hippocampal gyrus and subiculum of the rat. Acta Neuropathol 67:25–36
Wieloch T (1985) Hypoglycemia-induced neuronal damage prevented by an N-Methyl-d-aspartate antagonist. Science 230:681–683
Butcher SP, Jacobson I, Sandberg M, Hagberg H, Hamberger A (1987) 2-Amino-5-phosphonovalerate attenuates the severe hypoglycemia-induced loss of perforant path-evoked field potentials in the rat hippocampus. Neurosci Lett 76:296–300
Suh SW, Aoyama K, Chen Y, Garnier P, Matsumori Y, Gum E, Liu J, Swanson RA (2003) Hypoglycemic neuronal death and cognitive impairment are prevented by Poly (ADP-Ribose) Polymerase inhibitors administered after hypoglycemia. J Neurosci 33:10681–10690
Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA (2007) Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest 117:910–918
Suh SW, Hamby AM, Gum ET, Shin BS, Won SJ, Sheline CT, Chan PH, Swanson RA (2008) Sequential release of nitric oxide, zinc, and superoxide in hypoglycemic neuronal death. J Cereb Blood Flow Metab 28:1697–1706
Friberg H, Ferrand-Drake M, Bengtsson F, Halestrap AP, Wieloch T (1998) Cyclosporin A, but not FK 506, protects mitochondria and neurons against hypoglycemic damage and implicates the mitochondrial permeability transition in cell death. J Neurosci 14:5151–5159
Ferrand-Drake M, Zhu C, Gidö G, Hansen AJ, Karlsson JO, Bahr BA, Zamzami N, Kroemer G, Chan PH, Wieloch T, Blomgren K (2003) Cyclosporin A prevents calpain activation despite increased intracellular calcium concentrations, as well as translocation of apoptosis-inducing factor, cytochrome c and caspase-3 activation in neurons exposed to transient hypoglycemia. J Neurochem 85:1431–1442
Tong L, Perez-Polo R (1998) Brain-derived neurotrophic factor (BDNF) protects cultured rat cerebellar granule neurons against glucose deprivation-induced apoptosis. J Neural Transm 105:905–914
Xu Y, Zhang Q, Yu S, Yang Y, Ding F (2011) The protective effects of chitooligosaccharides against glucose deprivation-induced cell apoptosis in cultured cortical neurons through activation of PI3K/Akt and MEK/ERK1/2 pathways. Brain Res 1375:49–58
Rao RV, Bredesen DE (2004) Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr Opin Cell Biol 16:653–662
Michalak M, Parker JMR, Opas M (2002) Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium 32:269–278
Verkhratsky A (2005) Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev 85:201–279
Malhotra JD, Kaufman R (2007) The endoplasmic reticulum and the unfolded protein response. Sem Cell Dev Biol 18:716–731
Zhao L, Ackerman SL (2006) Endoplasmic reticulum stress in health and disease. Curr Opin Cell Biol 18:444–452
Merksamer PI, Papa FR (2010) The UPR and cell fate at a glance. J Cell Sci 123:1003–1006
Raghubir R, Nakka VP, Mehta SL (2011) Endoplasmic reticulum stress in brain damage. Methods Enzymol 489:259–275
Urano F, Wang XZ, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287:664–666
Weston CR, Davis RJ (2007) The JNK signal transduction pathway. Curr Opin Cell Biol 19:142–149
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nature Rev Mol Cell Biol 8:519–529
Lai E, Teodoro T, Volchuk A (2007) Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology 22:193–201
Boyce M, Yuan J (2006) Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ 13:363–373
Rasheva VI, Domingos P (2009) Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis 8:996–1007
Szegezdi E, Logue SE, Gorman AM, Samali A (2006) Mediators of endoplasmic reticulum stress induced apoptosis. EMBO Rep 7:880–885
Nakagawa T, Zhu H, Miroshima N, Li E, Xu J, Yankner BA, Yuan JY (2000) Caspase-12 mediated endoplasmic reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403:98–103
Nakagawa T, Yuan J (2000) Cross-talk between two cysteine protease families: activation of Caspase-12 by calpain in apoptosis. J Cell Biol 150:887–894
Rao RV, Hermel E, Castro-Obregon S, Del Rio G, Ellerby LM, Ellerby HM, Bredesen DE (2001) Coupling endoplasmic reticulum stress to the cell death program. Mechanism of caspase activation. J Biol Chem 276:33869–33874
Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, Katayama T, Tohyama M (2001) Activation of caspase-12, an endoplasmic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem 276:13935–13940
Paschen W, Aufenberg C, Hotop S, Mengesdorf T (2003) Transient cerebral ischemia activates processing of xbp1 messenger RNA indicative of endoplasmic reticulum stress. J Cereb Blood Flow Metab 23:449–461
Tajiri S, Oyadomari S, Yano S, Morioka M, Gotoh T, Hamada J-I, Ushio Y, Mori M (2004) Ischemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death Differ 11:403–415
Hayashi T, Saito A, Okuno S, Ferrand-Drake M, Dodd RL, Chan PH (2005) Damage to the endoplasmic reticulum and activation of apoptotic machinery by oxidative stress in ischemic neurons. J Cereb Blood Flow Metab 25:41–53
Morimoto N, Oida Y, Shimazawa M, Miura M, Kudo T, Imaizumi K, Hara H (2007) Involvement of endoplasmic reticulum stress after middle cerebral artery occlusion in mice. Neuroscience 147:957–967
Nakka VP, Gusain A, Raghubir R (2010) Endoplasmic reticulum stress plays critical role in brain damage after cerebral ischemia/reperfusion in rats. Neurotox Res 17:189–202
Benavides A, Pastor D, Santos P, Tranque P, Calvo S (2005) CHOP plays a pivotal role in the astrocyte death induced by oxygen and glucose deprivation. Glia 52:261–275
Badiola N, Penas C, Miñano-Molina A, Barneda-Zahonero B, Fadó R, Sánchez-Opazo G, Comella JX, Sabriá J, Zhu C, Blomgren K, Casas C, Rodríguez-Alvarez J (2011) Induction of ER stress in response to oxygen-glucose deprivation of cortical cultures involves the activation of the PERK and IRE-1 pathways and of caspase-12. Cell Death Dis 2:1–8
Mouw G, Zechel JL, Gamboa J, Lust WD, Selman WR, Ratcheson RA (2002) Activation of caspase-12, an endoplasmic reticulum resident caspase, after permanent focal ischemia in rat. J Mol Neurosci 14:183–186
Shibata M, Hattori H, Sasaki T, Gotoh J, Hamada J, Fukuuchi Y (2003) Activation of caspase-12 by endoplasmic reticulum stress induced by transient middle cerebral artery occlusion in mice. Neuroscience 118:491–499
Liu HJ, Yang JP, Wang CH, Liu RC, Li Y, Li CY (2009) Endoplasmic reticulum in the penumbra following middle cerebral artery occlusion in the rabbit. Neurol Sci 30:227–232
Hernández-Fonseca K, Massieu L, García de la Cadena S, Guzmán C, Camacho-Arroyo I (2012) Neuroprotective role of estradiol against neuronal death induced by glucose deprivation in cultured rat hippocampal neurons. Neuroendocrinology 96:41–50
Brewer GJ, Torricelli JR, Evege EK, Price PJ (1993) Optimized survival of hippocampal neurons in B27-suplemented neurobasal, a new serum free medium combination. J Neurosci Res 35:567–576
Mossmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Berridge MV, Tan AS (1993) Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch Biochem Biophys 303:474–482
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin-Phenol reagents. J Biol Chem 193:265–275
Miñano A, Caballero-Benitez A, Lluch M, Morán J, Rodríguez-Alvarez J (2008) C2-Ceramide mediates cerebellar granule cells apoptosis by activation of caspase-2, -9,-3. J Neurosci Res 86:1734–1747
Sokka AL, Putkonen N, Mudo G, Pryazhnikov E, Reijonen S, Khiroug L, Belluardo N, Lindholm D, Korhonen L (2007) Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci 27:901–908
Dahmer MK (2005) Caspases-2, -3, and -7 are involved in thapsigargin-induced apoptosis of SH-SY5Y neuroblastoma cells. J Neurosci Res 80:576–583
Larner SF, McKinsey DM, Hayes RL, Wang KKW (2005) Caspase 7: increased expression and activation after traumatic brain injury in rats. J Neurochem 94:97–108
Martinez JA, Zhang Z, Svetlov SI, Hayes RL, Wang KK, Larner SF (2010) Calpain and caspase processing of caspase-12 contribute to the ER stress-induced cell death pathway in differentiated PC12 cells. Apoptosis 15:1480–1493
Wek RC, Jiang HY, Anthony TG (2006) Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans 34:7–11
Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J (2005) A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science 307:935–939
Oyadomari S, Mori M (2004) Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11:381–389
Cryer PE (2006) Mechanisms of sympathoadrenal failure and hypoglycemia in diabetes. J Clin Invest 116:1470–1473
Sandberg M, Butcher SP, Hagberg H (1986) Extracellular overflow of neuroactive amino acids during severe insulin-induced hypoglycemia: in vivo dialysis of the rat hippocampus. J Neurochem 47:178–184
McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ (2001) Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol 21:1249–1259
Rao RV, Castro-Obregon S, Frankowski H, Schuler M, Stoka V, del Rio G, Bredesen DE, Ellerby HM (2002) Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway. J Biol Chem 277:21836–21842
Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y (2002) An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem 277:34287–34294
Mao W, Iwai C, Keng PC, Vulapalli R, Liang C (2006) Norepinephrine-induced oxidative stress causes PC-12 cell apoptosis by both endoplasmic reticulum stress and mitochondrial intrinsic pathway: inhibition of phosphatidylinositol 3-kinase survival pathway. Am J Physiol Cell Physiol 290:1373
Páramo B, Hernández-Fonseca K, Estrada-Sánchez AM, Jiménez N, Hernández-Cruz A, Massieu L (2010) Pathways involved in the generation of reactive oxygen and nitrogen species during glucose deprivation and its role on the death of cultured hippocampal neurons. Neuroscience 167:1057–1069
Zhang A, Zhang J, Sun P, Yao C, Su C, Sui T, Huang H, Cao X, Ge Y (2010) EIF2α and caspase-12 activation are involved in oxygen-glucose-serum deprivation/restoration-induced apoptosis of spinal cord astrocytes. Neurosci Lett 478:32–36
Malagelada C, Xifró X, Miñano A, Sabriá J, Rodríguez-Alvarez J (2005) Contribution of caspase-mediated apoptosis to cell death caused by oxygen-glucose deprivation in cortical cell cultures. Neurobiol Dis 20:27–37
Tan Y, Dourdin N, Wu C, De Veyra T, Elce JS, Greer PA (2006) Ubiquitous calpains promote caspase-12 and JNK activation during endoplasmic reticulum stress-induced apoptosis. J Biol Chem 281:16016–16024
Páramo B, Montiel T, Hernández-Espinosa DR, Rivera Martínez M, Morán J, Massieu L (2013) Calpain activation induced by glucose deprivation is mediated by oxidative stress and contributes to neuronal damage. Int J Biochem Cell Biol 45:2596–2604
Kumar R, Azam S, Sullivan JM, Owen C, Cavener DRC, Zhang P, Ron D, Harding HP, Chen JJ, Han A, White BC, Krause DJ, DeGracia DJ (2001) Brain ischemia and reperfusion activates the eukaryotic initiation factor 2α kinase, PERK. J Neurochem 77:1418–1421
Larner SF, Hayes RL, McKinsey DM, Pike BR, Wang KKW (2004) Increased expression and processing of caspase-12 after traumatic brain injury in rats. J Neurochem 88:78–90
Ibuki T, Yamasaki Y, Mizuguchi H, Sokabe M (2012) Protective effects of XBP1 against oxygen and glucose deprivation/reoxygenation injury in rat primary hippocampal neurons. Neurosci Lett 518:45–48
Reijonen S, Putkonen N, Nørremølle A, Lindholm D, Korhonen L (2008) Inhibition of endoplasmic reticulum stress counteracts neuronal cell death and protein aggregation caused by N-terminal mutant huntingtin proteins. Exp Cell Res 314:950–960
Saxena S, Cabuy E, Caroni P (2009) A role for motoneuron subtype–selective ER stress in disease manifestations of FALS mice. Nature Neurosci 12:627–636
Acknowledgments
This work was supported by S112179 CONACyT and IN211710-3 PAPIIT (UNAM) Grants to L.M. and 221026 CONACyT fellowship to S.G.C The authors thank Teresa Montiel for her technical assistance. This study was performed in partial fulfillment of the requirements for the PhD degree in Ciencias Bioquímicas of S. García de la Cadena at the Universidad Nacional Autónoma de México.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Inhibition of caspase-7, calpain and reticular stress suppresses caspase-12 processing. Caspase-12 proteolysis into its active fragment (36 kDa) was determined by Western blot. Cells were exposed to 1 h GD in the presence or the absence of DEVDCHO (25 μM), MDL-28170 (MDL, 50 μM) or salubrinal (50 μM). A representative Western blot is shown. Results represent mean ± SEM of three independent experiments and are expressed as the optical density of the active fragment (36 kDa) band/β-Actin (42 kDa). Data were analyzed by one-way ANOVA followed by a Fisher’s least significant difference test. * P < 0.05 versus 1 h GD and & P < 0.05 versus control. Supplementary material 1 (TIFF 12,198 kb)
Rights and permissions
About this article
Cite this article
de la Cadena, S.G., Hernández-Fonseca, K., Camacho-Arroyo, I. et al. Glucose deprivation induces reticulum stress by the PERK pathway and caspase-7- and calpain-mediated caspase-12 activation. Apoptosis 19, 414–427 (2014). https://doi.org/10.1007/s10495-013-0930-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-013-0930-7