Abstract
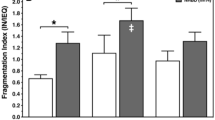
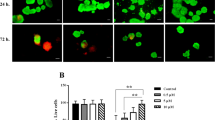
Human islet isolation is associated with adverse conditions inducing apoptosis and necrosis. The aim of the present study was to assess whether antiapoptotic preconditioning can improve in vitro and posttransplant function of isolated human islets. A dose-finding study demonstrated that 200 μmol/L of the caspase-3 inhibitor Ac-DEVD-CMK was most efficient to reduce the expression of activated caspase-3 in isolated human islets exposed to severe heat shock. Ac-DEVD-CMK-pretreated or sham-treated islets were transplanted into immunocompetent or immunodeficient diabetic mice and subjected to static glucose incubation to measure insulin and proinsulin secretion. Antiapoptotic pretreatment significantly deteriorated graft function resulting in elevated nonfasting serum glucose when compared to sham-treated islets transplanted into diabetic nude mice (p < 0.01) and into immunocompetent mice (p < 0.05). Ac-DEVD-CMK pretreatment did not significantly change basal and glucose-stimulated insulin release compared to sham-treated human islets but increased the proinsulin release at high glucose concentrations (20 mM) thus reducing the insulin-to-proinsulin ratio in preconditioned islets (p < 0.05). This study demonstrates that the caspase-3 inhibitor Ac-DEVD-CMK interferes with proinsulin conversion in preconditioned islets reducing their potency to cure diabetic mice. The mechanism behind this phenomenon is unclear so far but may be related to the ketone CMK linked to the Ac-DEVD molecule. Further studies are required to identify biocompatible caspase inhibitors suitable for islet preconditioning.




Similar content being viewed by others
References
Shapiro AM, Ricordi C, Hering BJ et al (2006) International trial of the Edmonton protocol for islet transplantation. N Engl J Med 355:1318–1330
Vantyghem MC, Kerr-Conte J, Arnalsteen L et al (2009) Primary graft function, metabolic control, and graft survival after islet transplantation. Diabetes Care 32:1473–1478
Bellin MD, Kandaswamy R, Parkey J et al (2008) Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes. Am J Transplant 8:2463–2470
Guignard AP, Oberholzer J, Benhamou PY et al (2004) Cost analysis of human islet transplantation for the treatment of type 1 diabetes in the Swiss-French Consortium GRAGIL. Diabetes Care 27:895–900
Kempf MC, Andres A, Morel P et al (2005) Logistics and transplant coordination activity in the GRAGIL Swiss-French multicenter network of islet transplantation. Transplantation 79:1200–1205
Caballero-Corbalan J, Brandhorst H, Malm H et al (2012) Using HTK for prolonged pancreas preservation prior to human islet isolation. J Surg Res 175:163–168
Johansson H, Lukinius A, Moberg L et al (2005) Tissue factor produced by the endocrine cells of the islets of langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes 54:1755–1762
Thomas FT, Contreras JL, Bilbao G, Ricordi C, Curiel D, Thomas JM (1999) Anoikis, extracellular matrix, and apoptosis factors in isolated cell transplantation. Surgery 126:299–304
Puthalakath H, Villunger A, O’Reilly LA et al (2001) Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science 293:1829–1832
Larsen CM, Wadt KA, Juhl LF et al (1998) Interleukin-1beta-induced rat pancreatic islet nitric oxide synthesis requires both the p38 and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases. J Biol Chem 273:15294–15300
Schliess F, Haussinger D (2002) The cellular hydration state: a critical determinant for cell death and survival. Biol Chem 383:577–583
Daoud JT, Petropavlovskaia MS, Patapas JM et al (2011) Long-term in vitro human pancreatic islet culture using three-dimensional microfabricated scaffolds. Biomaterials 32:1536–1542
Moritz W, Meier F, Stroka DM et al (2002) Apoptosis in hypoxic human pancreatic islets correlates with HIF-1alpha expression. FASEB J 16:745–747
Brandhorst H, Brandhorst D, Kumarasamy V, Maataoui A, Brendel MD, Bretzel RG (2003) Pretreatment of isolated islets with caspase-3 inhibitor DEVD increases graft survival after xenotransplantation. Transplant Proc 35:2142
Brandhorst H, Brandhorst D, Brendel MD, Hering BJ, Bretzel RG (1998) Assessment of intracellular insulin content during all steps of human islet isolation procedure. Cell Transplant 7:489–495
Ricordi C, Gray DW, Hering BJ et al (1990) Islet isolation assessment in man and large animals. Acta Diabetol Lat 27:185–195
Brandhorst H, Olbrich M, Neumann A, Jahr H, Brandhorst D (2007) Effect of pretransplant preconditioning by whole body hyperthermia on islet graft survival. Cell Transplant 16:707–715
Ris F, Hammar E, Bosco D et al (2002) Impact of integrin-matrix matching and inhibition of apoptosis on the survival of purified human beta-cells in vitro. Diabetologia 45:841–850
Berney T, Molano RD, Cattan P et al (2001) Endotoxin-mediated delayed islet graft function is associated with increased intra-islet cytokine production and islet cell apoptosis. Transplantation 71:125–132
Brandhorst D, Brandhorst H, Kumarasamy V et al (2003) Hyperthermic preconditioning protects pig islet grafts from early inflammation but enhances rejection in immunocompetent mice. Cell Transplant 12:859–865
Takada M, Nadeau KC, Hancock WW et al (1998) Effects of explosive brain death on cytokine activation of peripheral organs in the rat. Transplantation 65:1533–1542
Skrabal CA, Thompson LO, Potapov EV et al (2005) Organ-specific regulation of pro-inflammatory molecules in heart, lung, and kidney following brain death. J Surg Res 123:118–125
Contreras JL, Eckstein C, Smyth CA et al (2003) Brain death significantly reduces isolated pancreatic islet yields and functionality in vitro and in vivo after transplantation in rats. Diabetes 52:2935–2942
Saldeen J (2000) Cytokines induce both necrosis and apoptosis via a common Bcl-2-inhibitable pathway in rat insulin-producing cells. Endocrinology 141:2003–2010
Lakey JR, Rajotte RV, Warnock GL, Kneteman NM (1995) Human pancreas preservation prior to islet isolation. Cold ischemic tolerance. Transplantation 59:689–694
Hoeger S, Petrov K, Reisenbuechler A et al (2009) The additional detrimental effects of cold preservation on transplantation-associated injury in kidneys from living and brain-dead donor rats. Transplantation 87:52–58
Matsuda T, Suzuki Y, Tanioka Y et al (2003) Pancreas preservation by the 2-layer cold storage method before islet isolation protects isolated islets against apoptosis through the mitochondrial pathway. Surgery 134:437–445
Thomas D, Yang H, Boffa DJ et al (2002) Proapoptotic Bax is hyperexpressed in isolated human islets compared with antiapoptotic Bcl-2. Transplantation 74:1489–1496
Wang RN, Paraskevas S, Rosenberg L (1999) Characterization of integrin expression in islets isolated from hamster, canine, porcine, and human pancreas. J Histochem Cytochem 47:499–506
Stendahl JC, Kaufman DB, Stupp SI (2009) Extracellular matrix in pancreatic islets: relevance to scaffold design and transplantation. Cell Transplant 18:1–12
Rosenberg L, Wang R, Paraskevas S, Maysinger D (1999) Structural and functional changes resulting from islet isolation lead to islet cell death. Surgery 126:393–398
Krishnamurthy M, Li J, Fellows GF, Rosenberg L, Goodyer CG, Wang R (2011) Integrin {alpha}3, but not {beta}1, regulates islet cell survival and function via PI3 K/Akt signaling pathways. Endocrinology 152:424–435
Goto T, Tanioka Y, Sakai T et al (2007) Application of the two-layer method on pancreas digestion results in improved islet yield and maintained viability of isolated islets. Transplantation 83:754–758
Campbell PD, Weinberg A, Chee J et al (2012) Expression of pro- and antiapoptotic molecules of the bcl-2 family in human islets postisolation. Cell Transplant 21:49–60
Abdelli S, Ansite J, Roduit R et al (2004) Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes 53:2815–2823
Lucas-Clerc C, Massart C, Campion JP, Launois B, Nicol M (1993) Long-term culture of human pancreatic islets in an extracellular matrix: morphological and metabolic effects. Mol Cell Endocrinol 94:9–20
McConkey DJ (1998) Biochemical determinants of apoptosis and necrosis. Toxicol Lett 99:157–168
Emamaullee JA, Davis J, Pawlick R et al (2008) The caspase selective inhibitor EP1013 augments human islet graft function and longevity in marginal mass islet transplantation in mice. Diabetes 57:1556–1566
Nakano M, Matsumoto I, Sawada T et al (2004) Caspase-3 inhibitor prevents apoptosis of human islets immediately after isolation and improves islet graft function. Pancreas 29:104–109
Brandhorst D, Kumarasamy V, Maatoui A, Alt A, Bretzel RG, Brandhorst H (2006) Porcine islet graft function is affected by pretreatment with a caspase-3 inhibitor. Cell Transplant 15:311–317
Aikin R, Rosenberg L, Paraskevas S, Maysinger D (2004) Inhibition of caspase-mediated PARP-1 cleavage results in increased necrosis in isolated islets of Langerhans. J Mol Med 82:389–397
Prabhakaran K, Li L, Borowitz JL, Isom GE (2004) Caspase inhibition switches the mode of cell death induced by cyanide by enhancing reactive oxygen species generation and PARP-1 activation. Toxicol Appl Pharmacol 195:194–202
Piemonti L, Bertuzzi F, Nano R et al (1999) Effects of cryopreservation on in vitro and in vivo long-term function of human islets. Transplantation 68:655–662
Titus TT, Horton PJ, Badet L et al (2003) Adverse outcome of human islet-allogeneic blood interaction. Transplantation 75:1317–1322
Litterst CL (1980) Acute and subchronic toxicity of the protease inhibitor N-alpha-tosyl-L-lysyl-chloromethylketone (TLCK) in mice. Drug Chem Toxicol 3:227–235
Smith HJ (1978) Perspectives in the design of small molecule enzyme inhibitors as useful drugs. J Theor Biol 73:531–538
Lenzen S, Drinkgern J, Tiedge M (1996) Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 20:463–466
Tiedge M, Lortz S, Drinkgern J, Lenzen S (1997) Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 46:1733–1742
Author information
Authors and Affiliations
Corresponding author
Additional information
Daniel Brandhorst and Heide Brandhorst contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Brandhorst, D., Brandhorst, H., Maataoui, V. et al. Anti-caspase-3 preconditioning increases proinsulin secretion and deteriorates posttransplant function of isolated human islets. Apoptosis 18, 681–688 (2013). https://doi.org/10.1007/s10495-013-0834-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-013-0834-6