Abstract
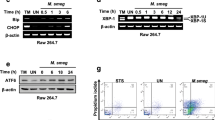
Although pathogenic mechanisms of tuberculosis have been extensively studied, little is known about the pathogenic mechanisms of Mycobacterium kansasii. In this work the influence of virulence and ER-stress mediated apoptosis of macrophages during two different strains of M. kansasii infection was investigated. We show that M. kansasii infection is associated with ER stress-mediated apoptosis in the murine macrophage cell line RAW 264.7. Infection of RAW 264.7 cells in vitro with apoptosis-inducing a clinical isolate of M. kansasii SM-1 (SM-1) resulted in strong induction of ER stress responses compared with M. kansasii type strain (ATCC 12478)-infected RAW 264.7 cells. Interestingly, inhibition of calpain prevented the induction of CHOP and Bip in ATCC 12478-infected RAW 264.7 cells but not in RAW 264.7 cells infected with SM-1. In contrast, reactive oxygen species (ROS) were significantly increased only in RAW 264.7 cells infected with SM-1. We propose that ROS generation is important for triggering ER stress-mediated apoptosis during SM-1 infection, whereas ATCC 12478-induced, ER stress-mediated apoptosis is associated with calpain activation. Our results demonstrate that the ER stress pathway plays important roles in the pathogenesis of M. kansasii infections, and that different strains of M. kansasii induce different patterns of ER stress-mediated apoptosis.






Similar content being viewed by others
References
Koh WJ, Kwon OJ, Jeon K, Kim TS, Lee KS, Park YK et al (2006) Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest 129:341–348
Bloch KC, Zwerling L, Pletcher MJ, Hahn JA, Gerberding JL, Ostroff SM et al (1998) Incidence and clinical implications of isolation of Mycobacterium kansasii: results of a 5-year, population-based study. Ann Intern Med 129:698–704
Marras TK, Daley CL (2002) Epidemiology of human pulmonary infection with nontuberculous mycobacteria. Clin Chest Med 23:553–567
Taillard C, Greub G, Weber R, Pfyffer GE, Bodmer T, Zimmerli S et al (2003) Clinical implications of Mycobacterium kansasii species heterogeneity: Swiss National Survey. J Clin Microbiol 41:1240–1244
Arend SM, Cerda de Palou E, de Haas P, Janssen R, Hoeve MA, Verhard EM et al (2004) Pneumonia caused by Mycobacterium kansasii in a series of patients without recognised immune defect. Clin Microbiol Infect 10:738–748
Evans SA, Colville A, Evans AJ, Crisp AJ, Johnston ID (1996) Pulmonary Mycobacterium kansasii infection: comparison of the clinical features, treatment and outcome with pulmonary tuberculosis. Thorax 51:1248–1252
Wieland CW, Florquin S, Pater JM, Weijer S, van der Poll T (2006) CD4+ cells play a limited role in murine lung infection with Mycobacterium kansasii. Am J Respir Cell Mol Biol 34:167–173
Santucci MB, Amicosante M, Cicconi R, Montesano C, Casarini M, Giosuè S et al (2000) Mycobacterium tuberculosis-induced apoptosis in monocytes/macrophages: early membrane modifications and intracellular mycobacterial viability. J Infect Dis 181:1506–1509
Dockrell DH, Lee M, Lynch DH, Read RC (2001) Immune-mediated phagocytosis and killing of Streptococcus pneumoniae are associated with direct and bystander macrophage apoptosis. J Infect Dis 184:713–722
Sohn H, Kim KW, Kang HB, Won CJ, Kim WS, Lee B et al (2010) Induction of macrophage death by clinical strains of Mycobacterium kansasii. Microb Pathog 48:160–167
Gan H, Lee J, Ren F, Chen M, Kornfeld H, Remold HG (2008) Mycobacterium tuberculosis blocks crosslinking of annexin-1 and apoptotic envelope formation on infected macrophages to maintain virulence. Nat Immunol 9:1189–1197
Park JS, Tamayo MH, Gonzalez-Juarrero M, Orme IM, Ordway DJ (2006) Virulent clinical isolates of Mycobacterium tuberculosis grow rapidly and induce cellular necrosis but minimal apoptosis in murine macrophages. J Leukoc Biol 79:80–86
Keane J, Remold HG, Kornfeld H (2000) Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J Immunol 164:2016–2020
Seimon TA, Kim MJ, Blumenthal A, Koo J, Ehrt S, Wainwright H et al (2010) Induction of ER stress in macrophages of tuberculosis granulomas. PLoS ONE 5:e12772
Song CH (2012) Endoplasmic reticulum stress responses and apoptosis. J Bacteriol Virol 42:196–202
Choi HH, Shin DM, Kang G, Kim KH, Park JB, Hur GM et al (2010) Endoplasmic reticulum stress response is involved in Mycobacterium tuberculosis protein ESAT-6-mediated apoptosis. FEBS Lett 584:2445–2454
Lim YJ, Choi JA, Choi HH, Cho SN, Kim HJ, Jo EK et al (2011) Endoplasmic reticulum stress pathway-mediated apoptosis in macrophages contributes to the survival of Mycobacterium tuberculosis. PLoS ONE 6:e28531
Shin DM, Jeon BY, Lee HM, Jin HS, Yuk JM, Song CH et al (2010) Mycobacterium tuberculosis eis regulates autophagy, inflammation, and cell death through redox-dependent signaling. PLoS Pathog 6:e1001230
Yang W, Tiffany-Castiglioni E, Koh HC, Son IH (2009) Paraquat activates the IRE1/ASK1/JNK cascade associated with apoptosis in human neuroblastoma SH-SY5Y cells. Toxicol Lett 191:203–210
Thornberry NA, Lazebnik Y (1998) Caspases: enemies within. Science 281:1312–1316
Masud A, Mohapatra A, Lakhani SA, Ferrandino A, Hakem R, Flavell RA (2007) Endoplasmic reticulum stress-induced death of mouse embryonic fibroblasts requires the intrinsic pathway of apoptosis. J Biol Chem 282:14132–14139
Nakagawa T, Yuan J (2000) Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol 150:887–894
Shao H, Chou J, Baty CJ, Burke NA, Watkins SC, Stolz DB et al (2006) Spatial localization of m-calpain to the plasma membrane by phosphoinositide biphosphate binding during epidermal growth factor receptor-mediated activation. Mol Cell Biol 26:5481–5496
Yang CS, Lee HM, Lee JY, Kim JA, Lee SJ, Shin DM et al (2007) Reactive oxygen species and p47phox activation are essential for the Mycobacterium tuberculosis-induced pro-inflammatory response in murine microglia. J Neuroinflammation 4:27
N’Diaye EN, Darzacq X, Astarie-Dequeker C, Daffe M, Calafat J, Maridonneau-Parini I (1998) Fusion of azurophil granules with phagosomes and activation of the tyrosine kinase Hck are specifically inhibited during phagocytosis of mycobacteria by human neutrophils. J Immunol 161:4983–4991
He S, Yaung J, Kim YH, Barron E, Ryan SJ, Hinton DR (2008) Endoplasmic reticulum stress induced by oxidative stress in retinal pigment epithelial cells. Graefes Arch Clin Exp Ophthalmol 246:677–683
Danelishvili L, McGarvey J, Li YJ, Bermudez LE (2003) Mycobacterium tuberculosis infection causes different levels of apoptosis and necrosis in human macrophages and alveolar epithelial cells. Cell Microbiol 5:649–660
Dolai S, Pal S, Yadav RK, Adak S (2011) Endoplasmic reticulum stress-induced apoptosis in Leishmania through Ca2+-dependent and caspase-independent mechanism. J Biol Chem 286:13638–13646
May KL, Paton JC, Paton AW (2010) Escherichia coli subtilase cytotoxin induces apoptosis regulated by host Bcl-2 family proteins Bax/Bak. Infect Immun 78:4691–4696
Wu J, Kaufman RJ (2006) From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ 13:374–384
Gallo KA, Johnson GL (2002) Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol 3:663–672
Tournier C, Dong C, Turner TK, Jones SN, Flavell RA, Davis RJ (2001) MKK7 is an essential component of the JNK signal transduction pathway activated by proinflammatory cytokines. Genes Dev 15:1419–1426
Shiraishi H, Okamoto H, Yoshimura A, Yoshida H (2006) ER stress-induced apoptosis and caspase-12 activation occurs downstream of mitochondrial apoptosis involving Apaf-1. J Cell Sci 119:3958–3966
Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, Katayama T et al (2001) Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem 276:13935–13940
Blomgren K, Zhu C, Wang X, Karlsson JO, Leverin AL, Bahr BA et al (2001) Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia: a mechanism of “pathological apoptosis”? J Biol Chem 276:10191–10198
Trimble WS, Grinstein S (2007) TB or not TB: calcium regulation in mycobacterial survival. Cell 130:12–14
Gonzalez-Cortes C, Reyes-Ruvalcaba D, Diez-Tascon C, Rivero-Lezcano OM (2009) Apoptosis and oxidative burst in neutrophils infected with Mycobacterium spp. Immunol Lett 126:16–21
Chen CC, Tsai SH, Lu CC, Hu ST, Wu TS, Huang TT et al (2012) Activation of an NLRP3 inflammasome restricts Mycobacterium kansasii infection. PLoS ONE 7:e36292
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) Grant funded by the Ministry of Education, Science and Technology (MEST) (2010-0008352), by the Ministry of Education, Science and Technology (MEST) (2010-0025985).
Conflict of interest
The authors have no financial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yun-Ji Lim and Hong-Hee Choi have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Lim, YJ., Choi, HH., Choi, JA. et al. Mycobacterium kansasii-induced death of murine macrophages involves endoplasmic reticulum stress responses mediated by reactive oxygen species generation or calpain activation. Apoptosis 18, 150–159 (2013). https://doi.org/10.1007/s10495-012-0792-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-012-0792-4