Abstract
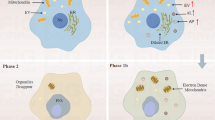
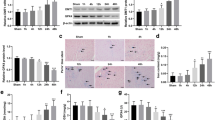
Endothelial cells (ECs) are directly exposed to hypoxia and contribute to injury during myocardial ischemia/reperfusion. Hypoxic preconditioning (HPC) protects ECs against hypoxia injury. This study aimed to explore whether HPC attenuates hypoxia/reoxygenation (H/R) injury by suppressing excessive endoplasmic reticulum stress (ERS) in cultured microvascular ECs (MVECs) from rat heart. MVECs injury was measured by lactate dehydrogenase (LDH) leakage, cytoskeleton destruction, and apoptosis. Expression of glucose regulating protein 78 (GRP78) and C/EBP homologous protein (CHOP), activation of caspase-12 (pro-apoptosis factors) and phosphorylation of p38 mitogen-activated protein kinase (p38 MAPK) were detected by western blot analysis. HPC attenuated H/R-induced LDH leakage, cytoskeleton destruction, and cell apoptosis, as shown by flow cytometry, Bax/Bcl-2 ratio, caspase-3 activation and terminal deoxynucleotidyl transferase mediated dUTP-biotin nick end labeling. HPC suppressed H/R-induced ERS, as shown by a decrease in expression of GRP78 and CHOP, and caspase-12 activation. HPC enhanced p38 MAPK phosphorylation but decreased that of protein kinase R-like ER kinase (PERK, upstream regulator of CHOP). SB202190 (an inhibitor of p38 MAPK) abolished HPC-induced cytoprotection, downregulation of GRP78 and CHOP, and activation of caspase-12, as well as PERK phosphorylation. HPC may protect MVECs against H/R injury by suppressing CHOP-dependent apoptosis through p38 MAPK mediated downregulation of PERK activation.









Similar content being viewed by others
Abbreviations
- CHOP:
-
CCAAT/enhancer-binding protein-homologous protein
- ER:
-
Endoplasmic reticulum
- ERS:
-
Endoplasmic reticulum stress
- GRP78:
-
Glucose regulating protein 78
- HPC:
-
Hypoxic preconditioning
- H/R:
-
Hypoxia/reoxygenation
- IPC:
-
Ischemic preconditioning
- I/R:
-
Ischemia/reperfusion
- LDH:
-
Lactate dehydrogenase
- MVECs:
-
Microvascular endothelial cells
- p38 MAPK:
-
p38 mitogen-activated protein kinase
- PDIA3:
-
Protein disulfide isomerase associated 3
- PERK:
-
Protein kinase R-like ER kinase
- TG:
-
Thapsigargin
- 2-DE:
-
Two-dimensional gel electrophoresis
References
Simionescu M (2007) Implications of early structural-functional changes in the endothelium for vascular disease. Arterioscler Thromb Vasc Biol 27:266–274
Liu XH, Wang S, Wu XD, Tang CS (2000) Association between delayed cardioprotection of aged rat myocytes is associated and activation of mitogen-activated protein kinase. Chin Med J 113:5–9
Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J (2003) Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 285:H579–H588
Liu XH, Zhang ZY, Sun S, Wu XD (2008) Ischemic postconditioning protects myocardium from ischemia/reperfusion injury through attenuating endoplasmic reticulum stress. Shock 30:422–427
Lehotský J, Urban P, Pavlíková M, Tatarková Z, Kaminska B, Kaplán P (2009) Molecular mechanisms leading to neuroprotection/ischemic tolerance: effect of preconditioning on the stress reaction of endoplasmic reticulum. Cell Mol Neurobiol 29:917–925
Nakamura K, Bossy-Wetzel E, Burns K, Lozyk M, Goping IS, Opas M, Bleackley RC, Green DR, Michalak M (2000) Changes in endoplasmic reticulum luminal environment affect cell sensitivity to apoptosis. J Cell Biol 150:731–740
Rao RV, Poksay KS, Castro-Obregon S, Schilling B, Row RH, del Rio G, Gibson BW, Ellerby HM, Bredesen DE (2004) Molecular components of a cell death pathway activated by endoplasmic reticulum stress. J Biol Chem 279:177–187
Xu C, Bailly-Maitre B, Reed J (2005) Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 115:2656–2664
Ron D (2002) Translational control in the endoplasmic reticulum stress response. J Clin Invest 110:1383–1388
Kumar S, Reusch HP, Ladilov Y (2008) Acidic pre-conditioning suppresses apoptosis and increases expression of Bcl-xL in coronary endothelial cells under simulated ischaemia. J Cell Mol Med 12:1584–1592
Gargalovic PS, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A, Truong A, Baruch-Oren T, Berliner JA, Kirchgessner TG, Lusis AJ (2006) The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol 26:2490–2496
Li Z, Zhang T, Dai H, Liu G, Wang H, Sun Y, Zhang Y, Ge Z (2008) Endoplasmic reticulum stress is involved in myocardial apoptosis of streptozocin-induced diabetic rats. J Endocrinol 196:565–572
Wu X, Liu X, Zhu X, Tang C (2007) Hypoxic preconditioning induces delayed cardioprotection through p38 MAPK-mediated calreticulin upregulation. Shock 27:572–577
Widmann C, Gibson S, Jarpe MB, Johnson GL (1999) Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev 79:143–180
Zhao TC, Hines DS, Kukreja RC (2001) Adenosine-induced late preconditioning in mouse hearts: role of p38 MAP kinase and mitochondrial KATP channels. Am J Physiol Heart Circ Physiol 280:H1278–H1285
Srivastava RK, Sollott SJ, Khan L, Hansford R, Lakatta EG, Longo DL (1999) Bcl-2 and Bcl-XL block thapsigargin-induced nitric oxide generation, c-Jun NH2-terminal kinase activity, and apoptosis. Mol Cell Biol 19:5659–5674
Liu XH, Wu XD, Cai LR, Sun S (2008) Calreticulin downregulation is associated with FGF-2-induced angiogenesis through calcineurin pathway in ischemic myocardium. Shock 29:140–148
Gillrie MR, Krishnegowda G, Lee K, Buret AG, Robbins SM, Looareesuwan S, Gowda DC, Ho M (2007) Src-family kinase_dependent disruption of endothelial barrier function by Plasmodium falciparum merozoite proteins. Blood 110:3426–3435
Zhang ZY, Liu XH, Hu WC, Rong F, Wu XD (2010) Calcineurin-myocyte enhancer factor 2c pathway mediates cardiac hypertrophy induced by endoplasmic reticulum stress in neonatal rat cardiomyocytes. Am J Physiol Heart Circ Physiol 298:H1499–H1509
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Sanchez JC, Rouge V, Pisteur M (1997) Improved and simplified in gel sample application using reswelling of dry immobilized pH gradients. Electrophoresis 18:324–327
Wang HC, Zhang HF, Guo WY, Su H, Zhang KR, Li QX, Yan W, Ma XL, Lopez BL, Christopher TA, Gao F (2006) Hypoxic postconditioning enhances the survival and inhibits apoptosis of cardiomyocytes following reoxygenation: role of peroxynitrite formation. Apoptosis 11:1453–1460
Papp Z, van der Velden J, Stienen GJM (2000) Calpain-induced alterations in the cytoskeletal structure and impaired mechanical properties of single myocytes of the rat. Cardiovasc Res 45:981–993
Natarajan R, Salloum FN, Fisher BJ, Smithson L, Almenara J, Fowler AA 3rd (2009) Prolyl hydroxylase inhibition attenuates post-ischemic cardiac injury via induction of endoplasmic reticulum stress genes. Vascul Pharmacol 51:110–118
Azfer A, Niu JL, Rogers LM, Adamski FM, Kolattukudy PE (2006) Activation of endoplasmic reticulum stress response during the development of ischemic heart disease. Am J Physiol Heart Circ Physiol 291:H1411–H1420
Schröder M, Kaufman RJ (2006) Divergent roles of IRE1alpha and PERK in the unfolded protein response. Curr Mol Med 6:5–36
Oyadomari S, Mori M (2004) Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11:381–389
Kim KM, Pae HO, Zheng M, Park R, Kim YM, Chung HT (2007) Carbon monoxide induces heme oxygenase-1 via activation of protein kinase R-like endoplasmic reticulum kinase and inhibits endothelial cell apoptosis triggered by endoplasmic reticulum stress. Circ Res 101:919–927
Friedman AD (1996) GADD153/CHOP, a DNA damage-inducible protein, reduced CAAT/enhancer binding protein activities and increased apoptosis in 32D c13 myeloid cells. Cancer Res 56:3250–3256
McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ (2001) Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol 21:1249–1259
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403:98–103
Mandlekar S, Kong AN (2001) Mechanisms of tamoxifen-induced apoptosis. Apoptosis 6:469–477
Ping P, Murphy E (2000) Role of p38 mitogen-activated protein kinases in preconditioning: a detrimental factor or a protective kinase? Circ Res 86:921–922
Hung CC, Ichimura T, Stevens JL, Bonventre JV (2003) Protection of renal epithelial cells against oxidativeinjury by endoplasmic reticulum stress preconditioning is mediated by ERK1/2 activation. J Biol Chem 278:29317–29326
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81070186) and a grant from the National Basic Research Program of China (No. 2011CB944004).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xu-Dong Wu and Zhen-Ying Zhang contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, XD., Zhang, ZY., Sun, S. et al. Hypoxic preconditioning protects microvascular endothelial cells against hypoxia/reoxygenation injury by attenuating endoplasmic reticulum stress. Apoptosis 18, 85–98 (2013). https://doi.org/10.1007/s10495-012-0766-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-012-0766-6