Abstract
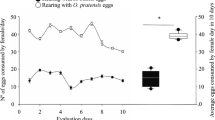
The Banks grass mite, Oligonychus pratensis (Banks) (Acari: Tetranychidae) causes significant damage to dates in California (USA), if not controlled. Studies are underway to develop biological control strategies against this pest in dates using the predatory mite Galendromus flumenis (Chant) (Acari: Phytoseiidae). In California date gardens, this predator is found in low numbers that are insufficient for the economic suppression of Banks grass mites, and our research aims to understand why it fails to keep up with prey densities. The hypothesis that prey density and predator interference interactively determine the predation efficiency of G. flumenis was tested. In addition, the effect of arena size and prey and predator density manipulations on the emigration rate of the predator was investigated. Our results indicate that the per capita predation rate of G. flumenis decreases steeply with increasing predator density due to mutual interference. Analysis of emigration data considering the arena size and predator numbers showed that the emigration rate of G. flumenis was higher from small arenas, and increased with increasing predator numbers. When emigration data were analyzed using prey and predator densities as independent variables, only the effect of predator density was significant, suggesting that higher predator density increases the emigration rate of G. flumenis. These results contribute to our understanding of the predator–prey interactions, and help in designing strategies for more efficient augmentative releases of G. flumenis.





Similar content being viewed by others
References
Abrams PA, Ginzburg LR (2000) The nature of predation: prey dependent, ratio dependent or neither? Trends Ecol Evol 15:337–341
Anderson JJ (2010) Ratio- and predator-dependent functional forms for predators optimally foraging in patches. Am Nat 175:240–249
Arditi R, Ginzburg LR (1989) Coupling in predator-prey dynamics: ratio-dependence. J Theor Biol 139:311–326
Arditi R, Ginzburg LR (2012) How species interact: altering the standard view of trophic ecology. Oxford University Press, New York
Arditi R, Akçakaya HR (1990) Understimation of mutual interference of predators. Oecologia 83:358–361
Banks N (1914) New mites. J Entomol Zool 6:1–57
Beddington JR (1975) Mutual interference between parasites or predators and its effect on searching efficiency. J Anim Ecol 44:331–340
Blackwood JS, Luh HK, Croft BA (2004) Evaluation of prey-stage preference as an indicator of life-style type in phytoseiid mites. Exp Appl Acarol 33:261–280
Clerc T, Davison AC, Bersier L-F (2009) Stochastic modelling of prey depletion processes. J Theor Biol 259:523–532
Crowley PH, Martin EK (1989) Functional-responses and interference within and between year classes of a dragonfly population. J N Am Benthol Soc 8:211–221
DeAngelis DL, Goldstein RA, O’Neil RV (1975) A model for trophic interaction. Ecology 56:881–892
Delong JP, Vasseur DA (2011) Mutual interference is common and mostly intermediate in magnitude. BMC Ecol 11:1–8
Elgar MA, Crespi BJ (1992) Cannibalism: ecology and evolution among diverse taxa. Oxford University Press, Oxford
Evans HF (1976) Mutual interference between predatory anthocorids. Ecol Entomol 1:283–286
Eveleigh ES, Chant DA (1982) Experimental studies on acarine predator-prey interactions: the effects of predator density on prey consumption, predator searching efficiency, and the functional response to prey density (Acarina: Phytoseiidae). Can J Zool 60:611–629
Farazmand A, Fathipour Y, Kamali K (2012) Functional response and mutual interference of Neoseiulus californicus and Typhlodromus bagdasarjani (Acari: Phytoseiidae) on Tetranychus urticae (Acari: Tetranychidae). Int J Acarol 38:369–376
Fryxell JM (2013) The great predator-prey debate. Ecology 94:1206–1207
Ganjisaffar F, Perring TM (2015a) Prey stage preference and functional response of the predatory mite Galendromus flumenis to Oligonychus pratensis. Biol Control 82:40–45
Ganjisaffar F, Perring TM (2015b) Relationship between temperature and development of Galendromus flumenis (Acari: Phytoseiidae), a predator of Banks grass mite (Acari: Tetranychidae). Exp Appl Acarol 67:535–546
Ganjisaffar F, Perring TM (2017) A life table analysis to evaluate biological control of Banks grass mite using the predatory mite, Galendromus flumenis (Acari: Phytoseiidae). Syst Appl Acarol 22:7–13
Hassell MP (1978) Arthropod predator-prey systems. Princeton University Press, Princeton
Hassell MP, Varley GC (1969) New inductive population model for insect parasites and its bearing on biological control. Nature 223:1113–1137
Henne DC, Johnson SJ (2010) Laboratory evaluation of aggregation, direct mutual interference, and functional response characteristics of Pseudacteon tricuspis Borgmeier (Diptera: Phoridae). Biol Control 55:63–71
Hoddle M, Van Driesche R, Sanderson J (1997) Biological control of Bemisia argentifolii (Homoptera: Aleyrodidae) on poinsettia with inundative releases of Encarsia formosa (Hymenoptera: Aphelinidae): are higher release rates necessarily better? Biol Control 10:166–179
Holling CS (1959) Some characteristics of simple types of predation and parasitism. Can Entomol 91:385–398
IBM SPSS Statistic (2016) SPSS 23.0 for windows, Chicago
Ivlev VS (1961) Experimental ecology of the feeding of fishes. Yale University Press, New Haven
Jensen CXJ, Ginzburg LR (2005) Paradoxes or theoretical failures? the jury is still out. Ecol Model 188:3–14
Jost C, Ellner SP (2000) Testing for predator dependence in predator-prey dynamics: a non-parametric approach. Proc R Soc Lond B 267:1611–1620
Juliano SA, Williams FM (1987) A comparison of methods for estimating the functional response parameters of the random predator equation. J Anin Ecol 56:641–653
Khodayari S, Fathipour Y, Sedaratian A (2016) Prey stage preference, switching and mutual interference of Phytoseius plumifer (Acari: Phytoseiidae) on Tetranychus urticae (Acari: Tetranychidae). Syst Appl Acarol 21:347–355
Kratina P, Vos M, Bateman A, Anholt BR (2009) Functional responses modified by predator density. Oecologia 159:425–433
Kuchlein JH (1966) Mutual interference among the predaceous mite Typhlodromus longipes Nesbitt (Acari: Phytoseiidae). 1. Effects of predator density on oviposition rate and migration tendency. Mededelingen Rijksfaculteit Landbouwwetenschappen Gent 31:740–745
McCoy MW, Stier AC, Osenberg CW (2012) Emergent effects of multiple predators on survival: the importance of depletion and the functional response. Ecol Lett 15:1449–1456
McMurtry JA, Scriven GT (1965) Insectary production of phytoseiid mites. J Econ Entomol 58:282–284
Nachman G (2006a) A functional response model of a predator population foraging in a patchy habitat. J Anim Ecol 75:948–958
Nachman G (2006b) The effects of prey patchiness, predator aggregation, and mutual interference on the functional response of Phytoseiulus persimilis feeding on Tetranychus urticae (Acari: Phytoseiidae, Tetranychidae). Exp Appl Acarol 38:87–111
Negm MW, De Moraes GJ, Perring TM (2015) Mite pests of date palms. In: Wakil W, Faleiro JR, Miller TA (eds) Sustainable pest management in date palm: current status and emerging challenges. Springer, Switzerland, pp 347–389
Nicholson AJ, Bailey VA (1935) The balance of animal populations. Proc Zool Soc Lond 105:551–598
Papanikolaou NE, Demiris N, Milonas PG, Preston S, Kypraios T (2016) Does mutual interference affect the feeding rate of Aphidophagous coccinellids? a modeling perspective. PLoS ONE 11:1–10
Reis PR, Sousa EO, Teodoro AV, Neto MP (2003) Effect of prey densities on the functional and numerical response of two species of predaceous mites (Acari: Phytoseiidae). Neotrop Entomol 32:461–467
Rogers D (1972) Random search and insect population models. J Anim Ecol 41:353–360
Royama T (1992) Analytical population dynamics. Chapman and Hall, London
SAS Institute (2014) SAS Enterprise Guide 7.1. SAS Institute Inc., Cary, North Carolina
Schausberger P (2003) Cannibalism among phytoseiid mites: a review. Exp Appl Acarol 29:173–191
Schmidt JM, Crist TO, Wrinn K, Rypstra AL (2014) Predator interference alters foraging behavior of a generalist predatory arthropod. Oecologia 175:501–508
Skalski GT, Gilliam JF (2001) Functional responses with predator interference: viable alternatives to the Holling type II model. Ecology 82:3083–3092
Skovgård H, Nachman G (2015) Effects of mutual interference on the ability of Spalangia cameroni (Hymenoptera: Pteromalidae) to attack and parasitize pupae of Stomoxys calcitrans (Diptera: Muscidae). Biol Control 44:1076–1084
Stephens DW, Brown JS, Ydenberg RC (2007) Foraging: behavior and ecology. University of Chicago Press, Chicago
Veeravel R, Baskaran P (1997) Searching behaviour of two coccinellid predators, Coccinella transversalis Fab. And Cheilomenes sexmaculatus Fab., on eggplant infested with Aphis gossypii Glow. Int J Trop Insect Sci 17:363–368
Wen B, Brower JH (1994) Suppression of Maize Weevil, Sitophilus zeamais (Coleoptera: Curculionidae), Populations in Drums of Corn by Single and Multiple Releases of the Parasitoid Anisopteromalus calandrae (Hymenoptera: Pteromalidae). J Kans Entomol Soc 67:331–339
Zhang Z-Q, Croft BA (1995) Intraspecific competition in immature Amblyseius fallacis, Amblyseius andersoni, Tvohlodromus occidentalis and Tvohlodromus pyri (Acari: Phytoseiidae). Exp Appl Acarol 19:65–77
Acknowledgements
We thank the many contributions of Albert Keck, Oscar Leal, Darcy Reed, and James Hepler. We are grateful to Drs. Timothy Paine, Subir Ghosh and Erin Rankin for providing useful comments on an earlier draft of this manuscript. This research was supported, in part, by the California Date Commission and Robert and Peggy van den Bosch Memorial Scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ganjisaffar, F., Nachman, G. & Perring, T.M. Mutual interference between adult females of Galendromus flumenis (Acari: Phytoseiidae) feeding on eggs of Banks grass mite decreases predation efficiency and increases emigration rate. Exp Appl Acarol 72, 1–14 (2017). https://doi.org/10.1007/s10493-017-0138-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-017-0138-6