Abstract
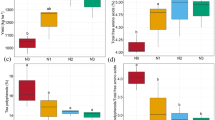
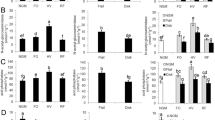
Agroforestry practice is believed to be an effective means of maintaining and improving soil fertility, and is widely used by farmers around the world. To gain better understanding of the effects of agroforestry practice on soil fertility, the organic carbon content, total nitrogen content, microbial biomass, basal respiration, and activity of soil enzymes at three soil depths (0–10, 10–20, and 20–30 cm) of Ginkgo (Ginkgo biloba L.)–tea (Camellia sinensis (L.) O. Kuntze) agroforestry systems were investigated. Study plots were established in Yushan Farm in Changshu, Jiangsu Province, China. These involved two densities of Ginkgo trees mixed with tea (G1 and G2) and monoculture tea systems (G0). The results showed that C, N, microbial biomass, and enzyme activity were higher in surface soil than in soil from the middle and lower layers whereas pH and metabolic quotient increased with soil depth. pH, microbial biomass C, N, basal respiration, and catalase and invertase activity in the 0–10 cm layer were significantly lower for G0 than for G1 and G2. Polyphenoloxidase activity in the 0–10 cm layer was significantly lower for G2 than for G0 and G1. Metabolic quotient in the 20–30 cm layer was significantly higher for G0 than for G2. The activity of soil enzymes, including catalase, dehydrogenase, urease, protease, and invertase, significantly and positively correlated with soil organic carbon and total nitrogen. The results of this study suggest that growing tea with Ginkgo could be regarded as good agroforestry practice which could enhance accumulation of organic matter in soil, improve the activity of soil enzymes, and maintain soil productivity and sustainability.


Similar content being viewed by others
References
Anderson TH, Domsch KH (1990) Application of eco-physiological quotients (qCO2 and qD) on microbial biomasses from soils of different cropping histories. Soil Biol Biochem 22:251–255
Balota EL, Chaves JC (2010) Enzymatic activity and mineralization of carbon and nitrogen in soil cultivated with coffee and green manures. Rev Bras Cienc Solo 34:1573–1583
Balota EL, Colozzi A, Andrade DS, Dick RP (2003) Microbial biomass in soils under different tillage and crop rotation systems. Biol Fert Soils 38:15–20
Berg MP, Kniese JP, Verhoef HA (1998) Dynamics and stratification of bacteria and fungi in the organic layers of a Scots pine forest soil. Biol Fert Soils 26:313–322
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Budiadi, Ishii HT, Sabarnurdin MS, Suryanto P, Kanazawa Y (2006) Biomass cycling and soil properties in an agroforestry-based plantation system of kayu putih (Melaleuca leucadendron LINN) in East Java, Indonesia. Agrofor Syst 67:135–145
Clegg CD (2006) Impact of cattle grazing and inorganic fertiliser additions to managed grasslands on the microbial community composition of soils. Appl Soil Ecol 31:73–82
De Costa WAJM, Surenthran P (2005) Tree-crop interactions in hedgerow intercropping with different tree species and tea in Sri Lanka: 1. Production and resource competition. Agrofor Forum 63:199–209
Fernandes SAP, Bettiol W, Cerri CC (2005) Effect of sewage sludge on microbial biomass, basal respiration, metabolic quotient and soil enzymatic activity. Appl Soil Ecol 30:65–77
Gu Y, Wang P, Kong CH (2009) Urease, invertase, dehydrogenase and polyphenoloxidase activities in paddy soil influenced by allelopathic rice variety. Eur J Soil Biol 45:436–441
Hamm D, Feger KH (1996) An optimized method for the determination of protease activity in acid forest soils. Z Pflanzenernähr Bodenkd 159:37–39
Hernández T, García C, Reinhardt I (1997) Short-term effect of wildfire on the chemical, biochemical and microbiological properties of Mediterranean pine forest soils. Biol Fert Soils 25:109–116
Islam KR, Weil RR (2000) Land use effects on soil quality in a tropical forest ecosystem of Bangladesh. Agr Ecosyst Environ 79:9–16
Jose S (2009) Agroforestry for ecosystem services and environmental benefits: an overview. Agrofor Syst 76:1–10
Keeney DR, Nelson DW (1982) Nitrogen-inorganic forms. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. American Society of Agronomy, Madison, pp 643–698
Klose S, Tabatabai MA (2000) Urease activity of microbial biomass in soils as affected by cropping systems. Biol Fert Soils 31:191–199
Kremer RJ, Li J (2003) Developing weed-suppressive soils through improved soil quality management. Soil Till Res 72:193–202
Lizarazo L, Jordá J, Juárez M, Sánchez-Andreu J (2005) Effect of humic amendments on inorganic N, dehydrogenase and alkaline phosphatase activities of a Mediterranean soil. Biol Fert Soils 42:172–177
Longo RM, de Melo WJ (2005) Urease activity in oxisols as influenced by vegetation cover and sampling time. Rev Bras Cienc Solo 29:645–650
Michel K, Matzner E (2003) Response of enzyme activities to nitrogen addition in forest floors of different C-to-N ratios. Biol Fert Soils 38:102–109
Mungai NW, Motavalli PP, Kremer RJ, Nelson KA (2005) Spatial variation of soil enzyme activities and microbial functional diversity in temperate alley cropping systems. Biol Fert Soils 42:129–136
Myers RT, Zak DR, White DC, Peacock A (2001) Landscape-level patterns of microbial community composition and substrate use in upland forest ecosystems. Soil Sci Soc Am J 65:359–367
Nancy WM, Peter PM (2005) Spatial variation of soil enzyme activities and microbial functional diversity in temperate alley cropping systems. Biol Fert Soils 42:129–136
Nioh I, Isobe T, Osada M (1993) Microbial biomass and some biochemical characteristics of a strongly acid tea field soil. Soil Sci Plant Nutr 39:617–626
Pandey A, Palni LMS (1996) The rhizosphere effect of tea on soil microbes in a Himalayan monsoonal location. Biol Fert Soils 21:131–137
Paudel BR, Udawatta RP, Anderson SH (2011) Agroforestry and grass buffer effects on soil quality parameters for grazed pasture and row-crop systems. Appl Soil Ecol 48:125–132
Perucci P, Bonciarelli U, Santilocchi R, Bianchi AA (1997) Effect of rotation, nitrogen fertilization and management of crop residues on some chemical, microbiological and biochemical properties of soil. Biol Fert Soils 24:311–316
Perucci P, Casucci C, Dumontet S (2000) An improved method to evaluate the o-diphenol oxidase activity of soil. Soil Biol Biochem 32:1927–1933
Schinner F, Von Mersi W (1990) Xylanase-, CM-cellulase-and invertase activity in soil: an improved method. Soil Biol Biochem 22:511–515
Shamir I, Steinberger Y (2007) Vertical distribution and activity of soil microbial population in a sandy desert ecosystem. Microb Ecol 53:340–347
Tian L, Dell E, Shi W (2010) Chemical composition of dissolved organic matter in agroecosystems: correlations with soil enzyme activity and carbon and nitrogen mineralization. Appl Soil Ecol 46:426–435
Udawatta RP, Kremer RJ, Adamson BW, Anderson SH (2008) Variations in soil aggregate stability and enzyme activities in a temperate agroforestry practice. Appl Soil Ecol 39:153–160
Udawatta RP, Kremer RJ, Garrett HE, Anderson SH (2009) Soil enzyme activities and physical properties in a watershed managed under agroforestry and row-crop systems. Agr Ecosyst Environ 131:98–104
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Wang G, Cao F (2011) Integrated evaluation of soil fertility in Ginkgo (Ginkgo biloba L.) agroforestry systems in Jiangsu, China. Agrofor Syst 83:89–100
Wang H, Huang Y, Huang H, Wang KM, Zhou SY (2005) Soil properties under young Chinese fir-based agroforestry system in mid-subtropical China. Agrofor Syst 64:131–141
Wardle DA (1992) A comparative assessment of factors which influence microbial biomass carbon and nitrogen levels in soil. Biol Rev 67:321–358
Wick B, Tiessen H, Menezes R (2000) Land quality changes following the conversion of the natural vegetation into silvo-pastoral systems in semi-arid NE Brazil. Plant Soil 222:59–70
Xue D, Yao H, Huang C (2006) Microbial biomass, N mineralization and nitrification, enzyme activities, and microbial community diversity in tea orchard soils. Plant Soil 288:319–331
Zhou LK (1987) Soil enzyme. Science Press, Beijing, pp 45–48
Zhou XG, Yu GB, Wu FZ (2011) Effects of intercropping cucumber with onion or garlic on soil enzyme activities, microbial communities and cucumber yield. Eur J Soil Biol 47:279–287
Acknowledgments
The authors thank the staff of Sanfeng Farm in Changshu, Jiangsu Province, China, for providing sites for the experiments, and for help and support during the study. Also, thanks to all the postgraduate students in the laboratory of silviculture in Nanjing Forestry University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tian, Y., Cao, F. & Wang, G. Soil microbiological properties and enzyme activity in Ginkgo–tea agroforestry compared with monoculture. Agroforest Syst 87, 1201–1210 (2013). https://doi.org/10.1007/s10457-013-9630-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-013-9630-0