Abstract
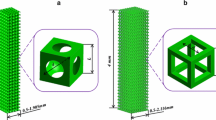
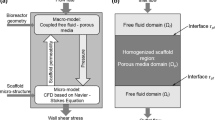
In this work, we investigated surface roughness effects on bone scaffold permeability and fluid flow-induced wall shear stress (WSS) using computational fluid dynamics (CFD) analysis. Scaffolds are made of interconnected microchannels, whose fluid flow can be examined from the perspective of fluid flow dynamics. Given that the roughness of microchannel surfaces serves a non-negligible function in the fluid dynamics within the channels, it is believed that the wall roughness of scaffolds can play an important role in their permeability and WSS. Given the criticality of permeability and WSS in the effective biological functioning of scaffolds, we investigated manufacturing-induced surface roughness effects on the two aforementioned biocompatibility characteristics. To this end, three scaffolds with square pores of different sizes (300, 600, and 900 µm) and identical porosity (63%) were designed. Six roughness levels (0, 4, 8, 12, 16, and 20 µm) were established for the scaffold walls, thus enabling us to develop 18 scaffold models. The pressure drop and WSS in the scaffolds were then measured by CFD. Scaffold permeability was calculated using Darcy’s law, with reference to geometrical parameters and the pressure drop derived from the CFD analysis. In all the scaffolds, high roughness decreased permeability and WSS. A significant difference in WSS reduction was found between the models with smooth scaffolds and the models with scaffolds that had a roughness of 20 µm. Except for the scaffold with a pore size of 300 µm, all the others showed no considerable change in permeability at different roughness levels.








Similar content being viewed by others
References
Abdalrahman, T., S. Scheiner, and C. Hellmich. Is trabecular bone permeability governed by molecular ordering-induced fluid viscosity gain? Arguments from re-evaluation of experimental data in the framework of homogenization theory. J. Theor. Biol. 365:433–444, 2015.
Ahmadi, S. M., S. A. Yavari, R. Wauthle, B. Pouran, J. Schrooten, H. Weinans, and A. A. Zadpoor. Additively manufactured open-cell porous biomaterials made from six different space-filling unit cells: the mechanical and morphological properties. Materials 8:1871–1896, 2015.
Ali, D., and S. Sen. Finite element analysis of the effect of boron nitride nanotubes in beta tricalcium phosphate and hydroxyapatite elastic modulus using the RVE model. Compos. Part B 90:336–340, 2016.
Ali, D., and S. Sen. Finite element analysis of boron nitride nanotubes’ shielding effect on the stress intensity factor of semielliptical surface crack in a wide range of matrixes using RVE model. Compos. Part B 110:351–360, 2017.
Ali, D., and S. Sen. Finite element analysis of mechanical behavior, permeability and fluid induced wall shear stress of high porosity scaffolds with gyroid and lattice-based architectures. J. Mech. Behav. Biomed. Mater. 75:262–270, 2017.
Ayati, B. P., C. M. Edwards, G. F. Webb, and J. P. Wikswo. A mathematical model of bone remodeling dynamics for normal bone cell populations and myeloma bone disease. Biol. Direct 5:28, 2010.
Bartnikowski, M., T. J. Klein, F. P. W. Melchels, and M. A. Woodruff. Effects of scaffold architecture on mechanical characteristics and osteoblast response to static and perfusion bioreactor cultures. Biotechnol. Bioeng. 111:1440–1451, 2014.
Blanquer, S. B. G., M. Werner, M. Hannula, S. Sharifi, G. P. R. Lajoinie, D. Eglin, J. Hyttinen, A. A. Poot, and D. W. Grijpma. Surface curvature in triply-periodic minimal surface architectures as a distinct design parameter in preparing advanced tissue engineering scaffolds. Biofabrication 9:025001, 2017.
Brindley, D., K. Moorthy, J.-H. Lee, C. Mason, H.-W. Kim, and I. Wall. Bioprocess forces and their impact on cell behavior: implications for bone regeneration therapy. J. Tissue Eng. 2011:620247, 2011.
Cavo, M., and S. Scaglione. Scaffold microstructure effects on functional and mechanical performance: integration of theoretical and experimental approaches for bone tissue engineering applications. Mater. Sci. Eng. C 68:872–879, 2016.
Chen, G. B., R. Xu, C. Zhang, and Y. G. Lv. Responses of MSCs to 3D scaffold matrix mechanical properties under oscillatory perfusion culture. ACS Appl. Mater. Interfaces. 9:1207–1218, 2017.
Conway, D. E., M. T. Breckenridge, E. Hinde, E. Gratton, C. S. Chen, and M. A. Schwartz. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr. Biol. 23:1024–1030, 2013.
Croce, G., and P. D’Agaro. Numerical simulation of roughness effect on microchannel heat transfer and pressure drop in laminar flow. J. Phys. D 38:1518–1530, 2005.
Dai, B. M., M. X. Li, and Y. T. Ma. Effect of surface roughness on liquid friction and transition characteristics in micro- and mini-channels. Appl. Therm. Eng. 67:283–293, 2014.
Dias, M. R., P. R. Fernandes, J. M. Guedes, and S. J. Hollister. Permeability analysis of scaffolds for bone tissue engineering. J. Biomech. 45:938–944, 2012.
Egan, P. F., S. J. Ferguson, and K. Shea. Design of hierarchical three-dimensional printed scaffolds considering mechanical and biological factors for bone tissue engineering. J. Mech. Des. 139:061401, 2017.
Gómez, S., M. D. Vlad, J. López, and E. Fernández. Design and properties of 3D scaffolds for bone tissue engineering. Acta Biomater. 42:341–350, 2016.
Graham, J. M., B. P. Ayati, S. A. Holstein, and J. A. Martin. The role of osteocytes in targeted bone remodeling: a mathematical model. PLoS ONE 8:10, 2013.
Guo, L., H. Xu, and L. Gong. Influence of wall roughness models on fluid flow and heat transfer in microchannels. Appl. Therm. Eng. 84:399–408, 2015.
Hendrikson, W. J., A. J. Deegan, Y. Yang, C. A. van Blitterswijk, N. Verdonschot, L. Moroni, and J. Rouwkema. Influence of additive manufactured scaffold architecture on the distribution of surface strains and fluid flow shear stresses and expected osteochondral cell differentiation. Front. Bioeng. Biotechnol. 5:6, 2017.
Kandlikar, S. G., D. Schmitt, A. L. Carrano, and J. B. Taylor. Characterization of surface roughness effects on pressure drop in single-phase flow in minichannels. Physics of Fluids 17:100606, 2005.
Kemppainen, J. M., and S. J. Hollister. Differential effects of designed scaffold permeability on chondrogenesis by chondrocytes and bone marrow stromal cells. Biomaterials 31:279–287, 2010.
Lesman, A., Y. Blinder, and S. Levenberg. Modeling of flow-induced shear stress applied on 3D cellular scaffolds: implications for vascular tissue engineering. Biotechnol. Bioeng. 105:645–654, 2010.
Lipowiecki, M., M. Ryvolova, A. Tottosi, N. Kolmer, S. Naher, S. A. Brennan, M. Vazquez, and D. Brabazon. Permeability of rapid prototyped artificial bone scaffold structures. J. Biomed. Mater. Res. Part A 102:4127–4135, 2014.
Marin, A. C., and D. Lacroix. The inter-sample structural variability of regular tissue-engineered scaffolds significantly affects the micromechanical local cell environment. Interface Focus 5:8, 2015.
Mitsak, A. G., J. M. Kemppainen, M. T. Harris, and S. J. Hollister. Effect of polycaprolactone scaffold permeability on bone regeneration in vivo. Tissue Eng. Part A 17:1831–1839, 2011.
Miyashita, S., N. E. M. B. Ahmed, M. Murakami, K. Iohara, T. Yamamoto, H. Horibe, K. Kurita, T. Takano-Yamamoto, and M. Nakashima. Mechanical forces induce odontoblastic differentiation of mesenchymal stem cells on three-dimensional biomimetic scaffolds. J. Tissue Eng. Regen. Med. 11:434–446, 2017.
Palacio, M. L. B., and B. Bhushan. Bioadhesion: a review of concepts and applications. Philos. Trans. R. Soc. A 370:2321–2347, 2012.
Palacio, M., S. Schricker, and B. Bhushan. Morphology and protein adsorption characteristics of block copolymer surfaces. J. Microsc. 240:239–248, 2010.
Pelević, N., and T. H. van der Meer. Heat transfer and pressure drop in microchannels with random roughness. Int. J. Therm. Sci. 99:125–135, 2016.
Rahbari, A., H. Montazerian, E. Davoodi, and S. Homayoonfar. Predicting permeability of regular tissue engineering scaffolds: scaling analysis of pore architecture, scaffold length, and fluid flow rate effects. Comput. Methods Biomech. Biomed. Eng. 20:231–241, 2017.
Rajendran, P., T. Rengarajan, J. Thangavel, Y. Nishigaki, D. Sakthisekaran, G. Sethi, and I. Nishigaki. The vascular endothelium and human diseases. Int. J. Biol. Sci. 9:1057–1069, 2013.
Rawool, A. S., S. K. Mitra, and S. G. Kandlikar. Numerical simulation of flow through microchannels with designed roughness. Microfluid. Nanofluid. 2:215–221, 2006.
Rumpler, M., A. Woesz, J. W. C. Dunlop, J. T. van Dongen, and P. Fratzl. The effect of geometry on three-dimensional tissue growth. J. R. Soc. Interface 5:1173–1180, 2008.
Santamaría, V. A. A., M. Malvè, A. Duizabo, A. M. Tobar, G. G. Ferrer, J. M. G. Aznar, M. Doblaré, and I. Ochoa. Computational methodology to determine fluid related parameters of non regular three-dimensional scaffolds. Ann. Biomed. Eng. 41:2367–2380, 2013.
Serpooshan, V., M. Julien, O. Nguyen, H. Wang, A. Li, N. Muja, J. E. Henderson, and S. N. Nazhat. Reduced hydraulic permeability of three-dimensional collagen scaffolds attenuates gel contraction and promotes the growth and differentiation of mesenchymal stem cells. Acta Biomater. 6:3978–3987, 2010.
Sonam, S., S. R. Sathe, E. K. F. Yim, M. P. Sheetz, and C. T. Lim. Cell contractility arising from topography and shear flow determines human mesenchymal stem cell fate. Sci. Rep. 6:20415, 2016.
Taylor, J. B., A. L. Carrano, and S. G. Kandlikar. Characterization of the effect of surface roughness and texture on fluid flow—past, present, and future. Int. J. Therm. Sci. 45:962–968, 2006.
Thompson, M. K. Methods for the Generation of Rough Surfaces in ANSYS. Cambridge: Mechanical Engineering Department, MIT, 2006.
Truscello, S., G. Kerckhofs, S. Van Bael, G. Pyka, J. Schrooten, and H. Van Oosterwyck. Prediction of permeability of regular scaffolds for skeletal tissue engineering: a combined computational and experimental study. Acta Biomater. 8:1648–1658, 2012.
Van Bael, S., Y. C. Chai, S. Truscello, M. Moesen, G. Kerckhofs, H. Van Oosterwyck, J. P. Kruth, and J. Schrooten. The effect of pore geometry on the in vitro biological behavior of human periosteum-derived cells seeded on selective laser-melted Ti6Al4V bone scaffolds. Acta Biomater. 8:2824–2834, 2012.
Voronov, R., S. VanGordon, V. I. Sikavitsas, and D. V. Papavassiliou. Computational modeling of flow-induced shear stresses within 3D salt-leached porous scaffolds imaged via micro-CT. J. Biomech. 43:1279–1286, 2010.
Vossenberg, P., G. A. Higuera, G. van Straten, C. A. van Blitterswijk, and A. J. B. van Boxtel. Darcian permeability constant as indicator for shear stresses in regular scaffold systems for tissue engineering. Biomech. Model. Mechanobiol. 8:499, 2009.
Wang, D., Y. Liu, Y. Q. Yang, and D. M. Xiao. Theoretical and experimental study on surface roughness of 316L stainless steel metal parts obtained through selective laser melting. Rapid Prototyp. J. 22:706–716, 2016.
Wang, G. L., D. W. Yang, Y. Wang, D. Niu, X. L. Zhao, and G. F. Ding. Heat transfer and friction characteristics of the microfluidic heat sink with variously-shaped ribs for chip cooling. Sensors 15:9547–9562, 2015.
Wittkowske, C., G. C. Reilly, D. Lacroix, and C. M. Perrault. In vitro bone cell models: impact of fluid shear stress on bone formation. Front. Bioeng. Biotechnol. 4:87, 2016.
Ali, D., and S. Sen. Permeability and fluid flow-induced wall shear stress of bone tissue scaffolds: computational fluid dynamic analysis using Newtonian and non-Newtonian blood flow models. Comput. Biol. Med. 99:201–208, 2018.
Yan, C., L. Hao, A. Hussein, and P. Young. Ti–6Al–4V triply periodic minimal surface structures for bone implants fabricated via selective laser melting. J. Mech. Behav. Biomed. Mater. 51:61–73, 2015.
Yousaf, M., and S. Usman. Natural convection heat transfer in a square cavity with sinusoidal roughness elements. Int. J. Heat Mass Transf. 90:180–190, 2015.
Zhao, F. H., T. J. Vaughan, and L. M. McNamara. Quantification of fluid shear stress in bone tissue engineering scaffolds with spherical and cubical pore architectures. Biomech. Model. Mechanobiol. 15:561–577, 2016.
Acknowledgments
This research has been supported by Ataturk University. The authors would like to thank the Ataturk University for funding and Dr. Kamil Kabakus for technical assistance.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ender Finol oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Ali, D., Sen, S. Computational Fluid Dynamics Study of the Effects of Surface Roughness on Permeability and Fluid Flow-Induced Wall Shear Stress in Scaffolds. Ann Biomed Eng 46, 2023–2035 (2018). https://doi.org/10.1007/s10439-018-2101-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-018-2101-z