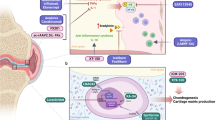
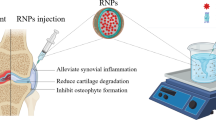
Abstract
Osteoarthritis (OA) is a progressive, degenerative disease of articulating joints that not only affects the elderly, but also involves younger, more active individuals with prolonged participation in high physical-demand activities. Thus, effective therapies that are easy to adopt clinically are critical in limiting the societal burden associated with OA. This review is focused on intra-articular injectable regimens and provides a comprehensive look at existing in vivo models of OA that might be suitable for developing, testing, and finding a cure for OA by intra-articular injections. We first discuss the pathology, molecular mechanisms responsible for the initiation and progression of OA, and challenges associated with disease-specific targeting of OA. We proceed to discuss available animal models of OA and provide a detailed perspective on the use of mouse models in studies of experimental OA. We finally provide a closer look at intra-articular injectable treatments for OA, focusing on biomaterials-based nanoparticles, and provide a comprehensive overview of the various nanometer-size ranges studied.




Similar content being viewed by others
References
Abramson, S. B. Osteoarthritis and nitric oxide. Osteoarthritis Cartilage 16(Suppl 2):S15–S20, 2008.
Arrich, J., et al. Intra-articular hyaluronic acid for the treatment of osteoarthritis of the knee: systematic review and meta-analysis. CMAJ 172:1039–1043, 2005.
Bajpayee, A. G., M. Scheu, A. J. Grodzinsky, and R. M. Porter. A rabbit model demonstrates the influence of cartilage thickness on intra-articular drug delivery and retention within cartilage. J. Orthop. Res. 33:660–667, 2015.
Bakker, A. C., et al. Prevention of murine collagen-induced arthritis in the knee and ipsilateral paw by local expression of human interleukin-1 receptor antagonist protein in the knee. Arthritis Rheum. 40:893–900, 1997.
Balazs, E. A., and J. L. Denlinger. Viscosupplementation—a new concept in the treatment of osteoarthritis. J. Rheumatol. 20:3–9, 1993.
Bannuru, R., N. Natov, U. Dasi, C. Schmid, and T. McAlindon. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis–meta-analysis. Osteoarthr. Cartil. 19:611–619, 2011.
Barve, R. A., et al. Transcriptional profiling and pathway analysis of monosodium iodoacetate-induced experimental osteoarthritis in rats: relevance to human disease. Osteoarthr. Cartil. 15:1190–1198, 2007.
Bellamy, N., et al. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Db. Syst. Rev. 2:CD005328, 2005.
Bishnoi, M., A. Jain, P. Hurkat, and S. K. Jain. Aceclofenac-loaded chondroitin sulfate conjugated SLNs for effective management of osteoarthritis. J. Drug Target. 22:805–812, 2014.
Bove, S. E., et al. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthr. Cartil. 11:821–830, 2003.
Boyce, B. F., and L. Xing. The RANKL/RANK/OPG pathway. Curr Osteoporos Rep 5:98–104, 2007.
Brown, T. D., R. C. Johnston, C. L. Saltzman, J. L. Marsh, and J. A. Buckwalter. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J. Orthop. Trauma 20:739–744, 2006.
Cashman, J. N. The mechanisms of action of NSAIDs in analgesia. Drugs 52(Suppl 5):13–23, 1996.
Chang, K. V., M. Y. Hsiao, W. S. Chen, T. G. Wang, and K. L. Chien. Effectiveness of intra-articular hyaluronic acid for ankle osteoarthritis treatment: a systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 94:951–960, 2013.
Chen, Z., et al. Hyaluronic acid-coated bovine serum albumin nanoparticles loaded with brucine as selective nanovectors for intra-articular injection. Int J Nanomed. 8:3843–3853, 2013.
Chen, Z., et al. Development of nanoparticles-in-microparticles system for improved local retention after intra-articular injection. Drug Deliv. 21:342–350, 2014.
Chevalier, X. Intraarticular treatments for osteoarthritis: new perspectives. Curr. Drug Targets 11:546–560, 2010.
Chevalier, X., et al. Safety study of intraarticular injection of interleukin 1 receptor antagonist in patients with painful knee osteoarthritis: a multicenter study. J. Rheumatol. 32:1317–1323, 2005.
Christiansen, B. A., et al. Musculoskeletal changes following non-invasive knee injury using a novel mouse model of post-traumatic osteoarthritis. Osteoarthr. Cartil. 20:773–782, 2012.
Coleman, P. J., D. Scott, J. Ray, R. M. Mason, and J. R. Levick. Hyaluronan secretion into the synovial cavity of rabbit knees and comparison with albumin turnover. J. Physiol. 503(Pt 3):645–656, 1997.
Cook, A. D., et al. Granulocyte-macrophage colony-stimulating factor is a key mediator in experimental osteoarthritis pain and disease development. Arthritis Res. Ther. 14:R199, 2012.
Cunnane, G., A. Madigan, E. Murphy, O. FitzGerald, and B. Bresnihan. The effects of treatment with interleukin-1 receptor antagonist on the inflamed synovial membrane in rheumatoid arthritis. Rheumatol. (Oxf.) 40:62–69, 2001.
Deberg, M., et al. One-year follow-up of Coll2-1, Coll2-1NO2 and myeloperoxydase serum levels in osteoarthritis patients after hip or knee replacement. Ann. Rheum. Dis. 67:168–174, 2008.
Esenyel, M., A. Icagasioglu, and C. Z. Esenyel. Effects of calcitonin on knee osteoarthritis and quality of life. Rheumatol. Int. 33:423–427, 2013.
Fang, H., and F. Beier. Mouse models of osteoarthritis: modelling risk factors and assessing outcomes. Nat. Rev. Rheumatol. 10:413–421, 2014.
Felson, D. T. Risk factors for osteoarthritis: understanding joint vulnerability. Clin Orthop Relat Res 427:S16–S21, 2004.
Fernandes, J., et al. In vivo transfer of interleukin-1 receptor antagonist gene in osteoarthritic rabbit knee joints: prevention of osteoarthritis progression. Am. J. Pathol. 154:1159–1169, 1999.
Fonseca, J. E., M. J. Santos, H. Canhao, and E. Choy. Interleukin-6 as a key player in systemic inflammation and joint destruction. Autoimmun. Rev. 8:538–542, 2009.
Furman, B. D., et al. Joint degeneration following closed intraarticular fracture in the mouse knee: a model of posttraumatic arthritis. J. Orthop. Res. 25:578–592, 2007.
Furman, B. D., et al. Targeting pro-inflammatory cytokines following joint injury: acute intra-articular inhibition of interleukin-1 following knee injury prevents post-traumatic arthritis. Arthritis Res. Ther. 16:R134, 2014.
Ghosh, P., and D. Guidolin. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: are the effects molecular weight dependent? Semin. Arthritis Rheum. 32:10–37, 2002.
Goldring, M. B., et al. Interleukin-1 beta-modulated gene expression in immortalized human chondrocytes. J. Clin. Invest. 94:2307–2316, 1994.
Gouze, E., et al. Lentiviral-mediated gene delivery to synovium: potent intra-articular expression with amplification by inflammation. Mol. Ther. 7:460–466, 2003.
Gupta, M., and G. M. Eisen. NSAIDs and the gastrointestinal tract. Curr. Gastroenterol. Rep. 11:345–353, 2009.
Henrotin, Y., M. Marty, and A. Mobasheri. What is the current status of chondroitin sulfate and glucosamine for the treatment of knee osteoarthritis? Maturitas 78:184–187, 2014.
Hochberg, M., X. Chevalier, Y. Henrotin, D. J. Hunter, and D. Uebelhart. Symptom and structure modification in osteoarthritis with pharmaceutical-grade chondroitin sulfate: what’s the evidence? Curr. Med. Res. Opin. 29:259–267, 2013.
Hochberg, M. C., et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthrit Care Res. 64:465–474, 2012.
Hong, S. L., and L. Levine. Inhibition of arachidonic acid release from cells as the biochemical action of anti-inflammatory corticosteroids. Proc. Natl. Acad. Sci. USA 73:1730–1734, 1976.
Hootman, J. M., and C. G. Helmick. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 54:226–229, 2006.
Huang, K., and L. D. Wu. Aggrecanase and aggrecan degradation in osteoarthritis: a review. J. Int. Med. Res. 36:1149–1160, 2008.
Hunter, D. J., and D. T. Felson. Osteoarthritis. Bmj 332:639–642, 2006.
Imamura, M., et al. Concentration of cytokines in patients with osteoarthritis of the knee and fibromyalgia. Clin. Interv. Aging 9:939–944, 2014.
Iqbal, I., and R. Fleischmann. Treatment of osteoarthritis with anakinra. Curr. Rheumatol. Rep. 9:31–35, 2007.
Ismail, H.M. et al. JNK2 controls aggrecan degradation in murine articular cartilage and the development of experimental osteoarthritis. Arthritis Rheumatol., 2015.
Jain, A., et al. Targeting of diacerein loaded lipid nanoparticles to intra-articular cartilage using chondroitin sulfate as homing carrier for treatment of osteoarthritis in rats. Nanomedicine 10:1031–1040, 2014.
Jevsevar, D. S. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J. Am. Acad. Orthop. Sur. 21:571–576, 2013.
Kang, M. L., and G. I. Im. Drug delivery systems for intra-articular treatment of osteoarthritis. Expert Opin. Drug Deliv. 11:269–282, 2014.
Kang, M. L., J. Y. Ko, J. E. Kim, and G. I. Im. Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials 35:9984–9994, 2014.
Kawashima, H., et al. Binding of a large chondroitin sulfate/dermatan sulfate proteoglycan, versican, to L-selectin, P-selectin, and CD44. J. Biol. Chem. 275:35448–35456, 2000.
Kay, J. D., et al. Intra-articular gene delivery and expression of interleukin-1Ra mediated by self-complementary adeno-associated virus. J. Gene Med. 11:605–614, 2009.
Killian, M. L., et al. Traumatic anterior cruciate ligament tear and its implications on meniscal degradation: a preliminary novel lapine osteoarthritis model. J. Surg. Res. 164:234–241, 2010.
Ko, F. C., et al. In vivo cyclic compression causes cartilage degeneration and subchondral bone changes in mouse tibiae. Arthritis Rheum. 65:1569–1578, 2013.
Kon, E., et al. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthroscopy 27:1490–1501, 2011.
Kruse, D. W. Intraarticular cortisone injection for osteoarthritis of the hip. Is it effective? Is it safe? Curr. Rev. Musculoskelet. Med. 1:227–233, 2008.
Lawrence, R. C., et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 41:778–799, 1998.
Little, C. B., et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 60:3723–3733, 2009.
Loeser, R. F. Molecular mechanisms of cartilage destruction: mechanics, inflammatory mediators, and aging collide. Arthritis Rheum. 54:1357–1360, 2006.
Malemud, C. J. Anticytokine therapy for osteoarthritis: evidence to date. Drugs Aging 27:95–115, 2010.
Martel-Pelletier, J., L. M. Wildi, and J. P. Pelletier. Future therapeutics for osteoarthritis. Bone 51:297–311, 2012.
McCoy, A. M. Animal Models of osteoarthritis: comparisons and key considerations. Vet. Pathol. 52:803–818, 2015.
Meheux, C. J., P. C. McCulloch, D. M. Lintner, K. E. Varner, and J. D. Harris. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy 32:495–505, 2016.
Miller, L. E., and J. E. Block. US-approved intra-articular hyaluronic acid injections are safe and effective in patients with knee osteoarthritis: systematic review and meta-analysis of randomized, saline-controlled trials. Clin. Med. Insights. Arthritis. Musculoskelet. Disord. 6:57–63, 2013.
Monticone, M. et al. Hyaluronic acid intra-articular Injection and exercise therapy: effects on pain and disability in subjects affected by lower limb joints osteoarthritis. The Italian Society of Physical and Rehabilitation Medicine (SIMFER) systematic review. Eur. J. Phys. Rehabil. Med., 2015.
Moreland, L. W. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res. Ther. 5:54–67, 2003.
Morgen, M., et al. Nanoparticles for improved local retention after intra-articular injection into the knee joint. Pharm. Res. 30:257–268, 2013.
Onur, T. S., et al. Joint instability and cartilage compression in a mouse model of posttraumatic osteoarthritis. J. Orthop. Res. 32:318–323, 2014.
Opal, S. M., and V. A. DePalo. Anti-inflammatory cytokines. Chest 117:1162–1172, 2000.
Pelletier, J. P., et al. In vivo suppression of early experimental osteoarthritis by interleukin-1 receptor antagonist using gene therapy. Arthritis Rheum. 40:1012–1019, 1997.
Perman, V. Clinical Biochemistry of Domestic Animals (3rd ed.). London: Academic Press, 1980.
Pi, Y., et al. Targeted delivery of non-viral vectors to cartilage in vivo using a chondrocyte-homing peptide identified by phage display. Biomaterials 32:6324–6332, 2011.
Pi, Y., et al. Intra-articular delivery of anti-Hif-2alpha siRNA by chondrocyte-homing nanoparticles to prevent cartilage degeneration in arthritic mice. Gene Ther. 22:439–448, 2015.
Pond, M. J., and G. Nuki. Experimentally-induced osteoarthritis in the dog. Ann. Rheum. Dis. 32:387–388, 1973.
Poulet, B., et al. Intermittent applied mechanical loading induces subchondral bone thickening that may be intensified locally by contiguous articular cartilage lesions. Osteoarthr. Cartil. 23:940–948, 2015.
Rampersad, R. R., et al. S100A9 is not essential for disease expression in an acute (K/BxN) or chronic (CIA) model of inflammatory arthritis. Scand. J. Rheumatol. 38:445–449, 2009.
Rothenfluh, D. A., H. Bermudez, C. P. O’Neil, and J. A. Hubbell. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nat. Mater. 7:248–254, 2008.
Rousseau, J., and P. Garnero. Biological markers in osteoarthritis. Bone 51:265–277, 2012.
Ryan, S. M., et al. An intra-articular salmon calcitonin-based nanocomplex reduces experimental inflammatory arthritis. J. Control Release 167:120–129, 2013.
Saklatvala, J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature 322:547–549, 1986.
Singh, A., et al. Nanoengineered particles for enhanced intra-articular retention and delivery of proteins. Adv. Healthc. Mater. 3:1562–1567, 2014.
Sommer, C., and M. Kress. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci. Lett. 361:184–187, 2004.
Steinbeck, M. J., L. J. Nesti, P. F. Sharkey, and J. Parvizi. Myeloperoxidase and chlorinated peptides in osteoarthritis: potential biomarkers of the disease. J. Orthop. Res. 25:1128–1135, 2007.
Takagi, H., Y. Asano, N. Yamakawa, I. Matsumoto, and K. Kimata. Annexin 6 is a putative cell surface receptor for chondroitin sulfate chains. J. Cell Sci. 115:3309–3318, 2002.
Wang, F., and X. He. Intra-articular hyaluronic acid and corticosteroids in the treatment of knee osteoarthritis: a meta-analysis. Exp. Ther. Med. 9:493–500, 2015.
Whitmire, R. E., et al. Self-assembling nanoparticles for intra-articular delivery of anti-inflammatory proteins. Biomaterials 33:7665–7675, 2012.
Williams, T. J., and M. J. Peck. Role of prostaglandin-mediated vasodilatation in inflammation. Nature 270:530–532, 1977.
Yang, C. C., C. Y. Lin, H. S. Wang, and S. R. Lyu. Matrix metalloproteases and tissue inhibitors of metalloproteinases in medial plica and pannus-like tissue contribute to knee osteoarthritis progression. PLoS ONE 8:e79662, 2013.
Yang, S., et al. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat. Med. 16:687–693, 2010.
Yasuda, T. Cartilage destruction by matrix degradation products. Mod. Rheumatol. 16:197–205, 2006.
Zhang, W., et al. EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann. Rheum. Dis. 64:669–681, 2005.
Zhang, W., et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr. Cartil. 16:137–162, 2008.
Zhou, Y., et al. In vivo anti-apoptosis activity of novel berberine-loaded chitosan nanoparticles effectively ameliorates osteoarthritis. Int. Immunopharmacol. 28:34–43, 2015.
Acknowledgments
The authors would like to thank the following funding agencies for supporting their research that was discussed in the review: NIH. Grant Numbers: R21-AR064034, R01-AG022021, RC4-AR060546, R01-AG028664, P30-AR046121. The authors would like to thank Dr. Mathias P. Bostrom at Hospital for Special Surgery, New York, for providing a de-identified knee joint image.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Associate Editor Akhilesh K Gaharwar oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Holyoak, D.T., Tian, Y.F., van der Meulen, M.C.H. et al. Osteoarthritis: Pathology, Mouse Models, and Nanoparticle Injectable Systems for Targeted Treatment. Ann Biomed Eng 44, 2062–2075 (2016). https://doi.org/10.1007/s10439-016-1600-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-016-1600-z