Abstract
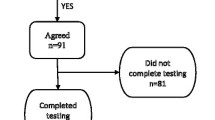
In this paper, we report on a new method for assisting in Meniere’s disease diagnosis. An accurate diagnosis of Meniere’s is challenging, and requires an expert opinion after observing several clinical assessments and tests over a period of time. Our proposed method is based on the analysis of the spontaneous and driven ear evoked responses recorded using Electrovestibulography (EVestG). We used the EVestG signals of 35 individuals suspected of Meniere’s and 26 age-matched healthy controls, out of which data of 14 patients with Meniere’s and 16 healthy controls were used for developing the diagnostic algorithm (training set) and the rest for testing. While recording and analyzing the test dataset, the researchers were only aware the patients suffered some dizziness, and were kept blind to the exact diagnoses till the end of study. EVestG field potentials (FPs) and their firing pattern, in response to several whole body tilt stimuli from both left and right ears were extracted. We investigated several features of the extracted FPs in response to each of side, back/forward, rotation, up/down, supine rotation, and supine up/down tilt stimulations, and selected the top five features showing the most significant differences between of the groups of the training set for every tilt. An ad-hoc average voting classifier was designed based on building five single-feature classifiers (using Linear Discriminant analysis) and taking the average of the single-feature classifiers’ votes. The results showed the side tilt data were best for the purpose of Meniere’s diagnosis; it resulted in 78% and 90% sensitivity and specificity for test dataset, respectively. The second best accuracy was achieved using back/forward tilt. The results and their implications are discussed. Overall, the EVestG side tilt results encourage the use of vestibular response as a non-invasive, robust and quick screening for Meniere’s and separating it from other types of dizziness.





Similar content being viewed by others
References
Asai, H., and N. Mori. Change in the summating potential and action potential during the fluctuation of hearing in Meniere’s disease. Scand. Audiol. 18(1):13–17, 1989.
Baloh, R. W., and V. Honrubia. Clinical Neurophysiology of the Vestibular System. New York: Oxford University Press, 2001.
Beasley, N. J., and N. S. Jones. Meniere’s disease: evolution of a definition. J. Laryngol. Otol. 110(12):1107–1113, 1996.
Blakley, B., Z. A. Dastgheib, B. Lithgow, and Z. Moussavi. Preliminary report: neural firing patterns specific for Meniere’s disease. J. Otolaryngol.-Head Neck Surg. 43(1):52, 2014.
Brown, D. J., Y. Chihara, and Y. Wang. Changes in utricular function during artificial endolymph injections in guinea pigs. Hear. Res. 304:70–76, 2013.
Chihara, Y., V. Wang, and D. J. Brown. Evidence for the utricular origin of the vestibular short-latency-evoked potential (VsEP) to bone-conducted vibration in guinea pig. Exp. Brain Res. 229(2):157–170, 2013.
Conlon, B. J., and W. P. Gibson. Electrocochleography in the diagnosis of Meniere’s disease. Acta Otolaryngol. 120:480–483, 2000.
Dastgheib, Z. A., B. Lithgow, B. Blakley, and Z. Moussavi. A new diagnostic vestibular evoked response. J. Otolaryngol.-Head Neck Surg. 44(1):14, 2015.
Dastgheib, Z. A., B. Lithgow, and Z. Moussavi, Application of fractal dimension on vestibular response signals for diagnosis of Parkinson’s disease. In: Conference of the IEEE Engineering in Medicine and Biology Society, pp. 7892–7895, 2011.
Duda, R. O., P. E. Hart, and D. G. Stork. Pattern Classification. New York: Wiley, 2001.
Ehtiati, T., W. Kinsner, and Z. Moussavi, Multifractal characterization of the electromyogram signals in presence of fatigue. In: IEEE Canadian Conference On Electrical and Computer Engineering, 1998, 1998, pp. 866–869.
Ferraro, J. A., Clinical electrocochleography: Overview of theories, techniques and applications. November 15, 2000; http://www.audiologyonline.com/articles/clinical-electrocochleography-overview-theories-techniques-1275-1275.
Ferraro, J. A. Electrocochleography: a review of recording approaches, clinical applications, and new findings in adults and children. J. Am. Acad. Audiol. 21(3):145–152, 2010.
Garrett A., D. Heibert, and B. Lithgow, Electrovestibulography: the “DC” potential used to separate Meniere’s disease and Benign Paroxysmal Positional Vertigo. In: Conference of the IEEE Engineering in Medicine and Biology Society, pp. 2381–2384, 2007.
Gnitecki, J., Z. Moussavi, and H. Pasterkamp, Classification of lung sounds during bronchial provocation using waveform fractal dimensions. In: 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2004. IEMBS’04, 2004, pp. 3844–3847.
Hain, T. C., Meniere’s Disease. http://www.dizziness-and-balance.com/disorders/menieres/menieres.html
Hearing, C. O., T. A. Balkany, G. A. Gates, et al. Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere’s disease*. Otolaryngol.-Head Neck Surg. 113:181–185, 1995.
Heibert, D., B. Lithgow, and K. Hourigan. Computer models of the vestibular head tilt response, and their relationship to EVestG and Meniere’s disease. World Acad. Sci. Eng. Technol. 4(5)(41):729–742, 2010.
Higuchi, T. Approach to an irregular time series on the basis of the fractal theory. Physica D 31(2):277–283, 1988.
Hogg, R., and J. Ledolter. Engineering Statistics. New York: Macmillan, 1987.
Kim, H. H., A. Kumar, R. A. Battista, et al. Electrocochleography in patients with Meniere’s disease. Am. J. Otolaryngol. 26(2):128–131, 2005.
Kinsner, L., Fractal and Chaos Engineering (Lecture Notes). Department of Electrical and Computer Engineering, Winnipeg, MB, Ch. 2 & 7, 2004.
Lithgow, B. J., A Neural Response System, Australia WO/2008/144840, June 31, 2007.
Lithgow, B. A methodology for detecting field potentials from the external ear canal: NEER and EVestG. Ann. Biomed. Eng. 40(8):1835–1850, 2012.
Lithgow, B. J., A. Garrett, and D. Heibert, EVestG: a measure for Meniere’s Disease. In: Conference of the IEEE Engineering in Medicine and Biology Society, pp. 4162–4165, 2008.
Mori, N., H. Asai, and M. Sakagami. The role of summating potential in the diagnosis and management of Meniere’s disease. Acta Otolaryngol. 113(s501):51–53, 1993.
Moscicki, E. K., E. F. Elkins, H. M. Baum, et al. Hearing loss in the elderly: an epidemiologic study of the Framingham Heart Study Cohort. Ear Hear. 6(4):184–190, 1985.
Noguchi, Y., H. Nishida, H. Tokano, et al. Comparison of acute low-tone sensorineural hearing loss versus Meniere’s disease by electrocochleography. Ann. Otol. Rhinol. Laryngol. 113(3 Pt 1):194–199, 2004.
Peng, H., F. Long, and C. Ding. Feature selection based on mutual information criteria of max-dependency, max-relevance, and min-redundancy. IEEE Trans. Pattern Anal. Mach. Intell. 27(8):1226–1238, 2005.
Selmani, Z., T. Marttila, and I. Pyykko. Incidence of virus infection as a cause of Meniere’s disease or endolymphatic hydrops assessed by electrocochleography. Eur. Arch. Otorhinolaryngol. 262(4):331–334, 2005.
Smith, C. A., H. Davis, B. H. Deatherage, et al. DC potentials of the membranous labyrinth. Am. J. Physiol. 193(1):203–206, 1958.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Nathalie Virag oversaw the review of this article.
Appendix
Appendix
Herein we explain the EVestG recording system more in details. In a typical EVestG experiment the subject sits on a hydraulic chair with eyes closed and head rested on the chair headrest while the electrodes already attached to him/her and to the chair. The hydraulic chair is placed inside a dark acoustically attenuated (>30 dB) and electromagnetically shielded chamber (Fig. 6).
The recording system with Hydraulic chair; the left and right ears’ signals are recorded using Spike2 software via a CED-1902 amplifier (50 or 60 Hz notch filter (for Australia and Canada, respectively), 20 k gain, 1 Hz high pass filter) and digitized using CED1401 ADC board at sampling rate of 41,666 Hz.
In order to stimulate the vestibular system, sinusoidal whole body tilts are delivered via a computer that controls hydraulic chair. The hydraulic chair can passively move the body and consequently the head in a way to resemble the head‘s pitch, yaw, or roll motions as well as a linear up and down translations. As mentioned before, subjects receive seven whole body tilting stimuli. These stimuli are: 15 cm upward movement of the chair, while the subject is in upright sitting position (up/down tilt), 40° rotation of the chair to the right side, either in upright sitting position (rotation tilt) or in supine position (supine rotation), 40° tilting of the chair to the right side (IT right and CT left tilts), 40° tilting of the chair to the left side (IT left and CT right tilts) and 40° tilting of the chair backward (back/forward tilt). Each of these movements can selectively and predominantly stimulate components of the vestibular sensory organ, which is sensitive to motion in that direction.
The chair movement position (for 40° tilt) and its velocity profile are shown in Fig. 7. Using the hydraulic system enables the chair in achievement of uniform and smooth sinusoidal movements in terms of velocity and position during the recordings. For every tilt the chair movement (to or from the tilting position) has acceleration and deceleration phases which each take 1.5 s and are called OnAA and OnBB respectively.
Position and velocity profiles of the vestibular stimulus. Acceleration and deceleration of the chair movement (1st and 2nd 1.5 s) are separated by the dashed vertical line.19
As an example, side tilt stimulus has duration of 120 s in which the first 60 s is dedicated to tilting of the chair to the right side and the next 60 s is dedicated to tilting of the chair to the left side. During the side tilt and when the chair tilts to the right side, IT right and CT left ear signals are recorded whilst the IT left and CT right ear signals are recorded when the chair tilts to the left side.
During the experiment, the position waveform of the chair is recorded simultaneously with the raw EVestG signals from left and right ears (Fig. 8). It was used for synchronization of the segments of interest for analysis. The periods of interest in each tilt are 1.5 s before the movement labeled as background (BGi), the 3 s tilting stimuli (OnAA and OnBB), the 1.5 s before returning the chair to background position (return to center BGi or RTC BGi), and the 3 s stimuli following that (RTC OnAA and RTC OnBB). The acceleration/deceleration segments are selected as they give the largest differences compared to background. EVestG signals were recorded at a sampling rate of 41666 Hz. After the recordings, ear signals along with the background noise are fed to the NEER algorithm24 to extract two main signals: average FP and its firing pattern for each segment. The reliability of EVestG system is like the reliability of any other electronic measuring device and there is no inconsistency from one subject’s data to another.
The chair movement pattern during the side tilt. The side tilt stimulus has a duration of 120 s; it begins with a 20 s background recording, with the subject sitting in a still position without any inclination, followed by a 3 s tilt to the right (about 40°), 17 s rest in the tilted position, 3 s moving back to center, 37 s rest at the center position, 3 s tilt to the left, 17 s rest at the left position, 3 s return to the center and 17 s rest at the center.
Rights and permissions
About this article
Cite this article
Dastgheib, Z.A., Lithgow, B., Blakely, B. et al. Application of Vestibular Spontaneous Response as a Diagnostic Aid for Meniere’s Disease. Ann Biomed Eng 44, 1672–1684 (2016). https://doi.org/10.1007/s10439-015-1441-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-015-1441-1