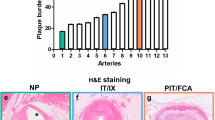
Abstract
Coronary angioplasty initially employed balloon dilatation only. This technique revolutionized the treatment of coronary artery disease, although outcomes were compromised by acute vessel closure, late constrictive remodeling, and restenosis due to neointimal proliferation. These processes were studied in animal models, which contributed to understanding the biology of endovascular arterial injury. Coronary stents overcome acute recoil, with improvements in the design and metallurgy since then, leading to the development of drug-eluting stents and bioresorbable scaffolds. These devices now undergo computer modeling and benchtop and animal testing before evaluation in clinical trials. Animal models, including rabbit, sheep, dog and pig are available, all with individual benefits and limitations. In smaller mammals, such as mouse and rabbit, the target for stenting is generally the aorta; whereas in larger animals, such as the pig, it is generally the coronary artery. The pig coronary stenting model is a gold-standard for evaluating safety; but insights into biomechanical properties, the biology of stenting, and efficacy in controlling neointimal proliferation can also be gained. Intra-coronary imaging modalities such as intravascular ultrasound and optical coherence tomography allow precise serial evaluation in vivo, and recent developments in genetically modified animal models of atherosclerosis provide realistic test beds for future stents and scaffolds.




Similar content being viewed by others
References
Ali, Z. A., N. J. Alp, H. Lupton, N. Arnold, T. Bannister, Y. Hu, S. Mussa, M. Wheatcroft, D. Greaves, J. Gunn, and K. Channon. Increased in-stent stenosis in ApoE knockout mice: insights from a novel mouse model of balloon angioplasty and stenting. Arterioscler. Thromb. Vasc. Biol. 27:833–840, 2007.
Balakrishnan, B., A. R. Tsafriri, P. Seifert, A. Groothuis, C. Rogers, and E. R. Edelman. Strut position, blood flow, and drug deposition: implications for single and overlapping drug-eluting stents. Circulation 111:2958–2965, 2005.
Bauters, C., T. Meurice, M. Hamon, E. McFadden, J. M. Lablanche, and M. E. Bertrand. Mechanisms and prevention of restenosis: from experimental models to clinical practice. Cardiovasc. Res. 31:835–846, 1996.
Caiazzo, A., D. Evans, J.-L. Falcone, J. Hegewald, E. Lorenz, B. Stahl, D. Wang, J. Bernsdorf, B. Chopard, J. Gunn, R. Hose, M. Krafczyk, P. Lawford, R. Smallwood, D. Walker, and A. Hoekstra. A complex automata approach for in-stent restenosis: two-dimensional multiscale modeling and simulations. J Comput. Sci. 2:9–17, 2011.
Caro, C. G., A. Seneviratne, K. B. Heraty, C. Monaco, M. G. Burke, R. Krams, C. C. Chang, P. Gilson, and G. Coppola. Intimal hyperplasia following implantation of helical centreline and straight centreline stents in common carotid arteries in healthy pigs: influence of intraluminal flow. J. R. Soc. Interface 10(89):20130578, 2013.
Carrozza, J. P., S. E. Hosley, D. J. Cohen, and D. S. Baim. In vivo assessment of stent expansion and recoil in normal porcine coronary arteries: differential outcome by stent design. Circulation 100:756–760, 1999.
Carter, A. J., M. Aggarwal, G. A. Kopia, F. Tio, T. S. Tsao, R. Kolata, A. C. Yeung, G. Llanos, J. Dooley, and R. Falotico. Long term effects of polymer-based, slow release, sirolimus-eluting stents in a porcine coronary model. Cardiovasc. Res. 63:617–624, 2004.
Carter, A. J., J. R. Laird, A. Farb, W. Kufs, D. C. Wortham, and R. Virmani. Morphologic characteristics of lesion formation and time course of smooth muscle cell proliferation in a porcine proliferative restenosis model. J. Am. Coll. Cardiol. 24:1398–1405, 1994.
Carter, A. J., J. R. Laird, W. M. Kufs, L. Bailey, T. G. Hoopes, T. Reeves, A. Farb, and R. Virmani. Coronary stenting with a novel stainless steel balloon-expandable stent: determinants of neointimal formation and changes in arterial geometry after placement in an atherosclerotic model. J. Am. Coll. Cardiol. 27:1270–1277, 1996.
Carter, A. J., D. Scott, D. Rahdert, L. Bailey, J. De Vries, K. Ayerdi, T. Turnlund, R. Jones, R. Virmani, and T. A. Fischell. Stent design favourably influences the vascular response in normal porcine coronary arteries. J. Invasive Cardiol. 11:127–134, 1999.
Chamberlain, J., M. Wheatcroft, N. Arnold, H. Lupton, D. C. Crossman, J. Gunn, and S. Francis. A novel mouse model of in situ stenting. Cardiovasc. Res. 85:38–44, 2010.
Curtin, A. E., and L. Zhou. An agent-based model of the response to angioplasty and bare metal stent deployment in an atherosclerotic blood vessel. PLoS One 9:e9441, 2014.
De Meyer, S. F., S. Staelens, P. N. Badenhorst, H. Pieters, S. Lamprecht, J. Roodt, S. Janssens, M. Meiring, K. Vanhoorelbeke, A. Bruwer, S. Brown, and H. Deckmyn. Coronary artery in-stent stenosis persists despite inhibition of the von Willebrand factor-collagen interaction in baboons. Thromb. Haemost. 98:1343–1349, 2007.
Dean, C. J., A. C. Morton, N. D. Arnold, D. R. Hose, D. C. Crossman, and J. Gunn. Relative importance of the components of stent geometry to stretch-induced in-stent neointima formation. Heart 91:1603–1604, 2005.
Finn, A. V., H. K. Gold, A. Tang, D. K. Weber, T. N. Wight, A. Clermont, R. Virmani, and F. D. Kolodgie. A novel rat model of carotid artery stenting for the understanding of restenosis in metabolic diseases. J. Vasc. Res. 39:414–425, 2002.
Gallo, R., A. Padurean, T. Jayaraman, S. Marx, M. Roque, S. Adelman, J. Chesebro, J. Fallon, V. Fuster, A. Marks, and J. J. Badimon. Inhibition of intimal thickening after balloon angioplasty in porcine coronary arteries by targeting regulators of the cell cycle. Circulation 99:2164–2170, 1999.
Garasic, J. M., E. R. Edelman, J. C. Squire, P. Seifert, M. S. Williams, and C. Rogers. Stent and artery geometry determine intimal thickening independent of arterial injury. Circulation 101:812–818, 2000.
Grinstead, W. C., G. P. Rodgers, W. Mazur, B. A. French, D. Cromeens, C. Van Pelt, S. M. West, and A. E. Raizner. Comparison of three porcine restenosis models: the relative importance of hypercholesterolemia, endothelial abrasion, and stenting. Coron. Art. Dis. 5:425–434, 1994.
Grüntzig, A., H. H. Riedhammer, M. Turina, and W. Rutishauser. A new method for the percutaneous dilation of coronary stenoses in animal experiment. Verh. Dtsch. Ges. Kreislaufforsch. 42:282–285, 1976.
Gunn, J., K. H. Chan, N. Arnold, L. Shepherd, D. Cumberland, and D. Crossman. Coronary artery stretch vs. deep injury in the development of in-stent neointima. Heart 88:401–405, 2002.
Gupta, G. K., K. Dhar, M. G. Del Core, W. J. Hunter, 3rd, G. I. Hatzoudis, and D. K. Agrawal. Suppressor of cytokine signaling-3 and intimal hyperplasia in porcine coronary arteries following coronary intervention. Exp. Mol. Pathol. 91(1):346–352, 2011.
Hamamdzic, D., and R. L. Wilensky. Porcine models of accelerated coronary atherosclerosis: role of diabetes mellitus and hypercholesterolemia. J. Diabetes Res. 2013:761415, 2013.
Hehrlein, C., M. Zimmermann, J. Pill, J. Metz, W. Kuebler, and E. von Hodenberg. The role of elastic recoil after balloon angioplasty of rabbit arteries and its prevention by stent implantation. Eur. Heart J. 15:277–280, 1994.
Heublein, B., R. Rohde, V. Kaese, M. Niemeyer, W. Hartung, and A. Haverich. Biocorrosion of Magnesium alloys: a new principle in cardiovascular implant technology? Heart 89:651–656, 2003.
Hong, M. K., R. Beyar, R. Kornowski, F. O. Tio, O. Bramwell, and M. B. Leon. Acute and chronic effects of self-expanding nitinol stents in porcine coronary arteries. Coron. Art. Dis. 8:45–48, 1997.
Iqbal, J., J. Gunn, and P. W. Serruys. Coronary stents: historical development, current status and future directions. Br. Med. Bull. 106:193–211, 2013.
Kelly, D., A. Morton, N. Arnold, J. Mecinovic, C. Schofield, H. Lupton, K. Al-Lamee, D. Crossman, J. Gunn, and A. Gershlick. Activation of hypoxia-inducible factor by di-methyl oxalyl glycine (DMOG) increases neovascularisation but not functional vascular supply within ischemic myocardium in a porcine coronary artery occlusion model. J. Clin. Exp. Cardiol. 2:8, 2011.
Krupski, W. C., A. Bass, A. B. Kelly, U. M. Marzec, S. R. Hanson, and L. A. Harker. Heparin-resistant thrombus formation by endovascular stents in baboons. Interruption by a synthetic antithrombin. Circulation 82:570–577, 1990.
LaDisa, J. F., LE Olson, H. A. Douglas, D. C. Warltier, J. R. Kersten, and P. S. Pagel. Alterations in regional vascular geometry produced by theoretical stent implantation influence distributions of wall shear stress: analysis of a curved coronary artery using 3D computational fluid dynamics modelling. Biomed. Eng. Online 5:40, 2006.
LaDisa, J. F., L. E. Olson, I. Guler, D. A. Hettrick, S. H. Audi, J. R. Kersten, D. C. Warltier, and P. S. Pagel. Stent design properties and deployment ratio influence indexes of wall shear stress: a three-dimensional computational fluid dynamics investigation within a normal artery. J. Appl. Physiol. 97:424–430, 2004.
Lambert, T. L., V. Dev, E. Rechavia, J. S. Forrester, F. Litvack, and N. L. Eigler. Localized arterial wall drug delivery from a polymer coated removable metallic stent. Kinetics, distribution, and bioactivity of forskolin. Circulation 90:1003–1011, 1994.
Lane, J. P., L. E. Perkins, A. J. Sheehy, E. J. Pacheco, M. P. Frie, B. J. Lambert, R. J. Rapoza, and R. Virmani. Lumen gain and restoration of pulsatility after implantation of a bioresorbable vascular scaffold in porcine coronary arteries. J. Am. Coll. Cardiol. Cardiovasc. Intervent. 7:688–695, 2014.
Langeveld, B., A. J. Roks, R. A. Tio, A. J. van Boven, J. J. van der Want, R. H. Henning, H. M. van Beusekom, W. J. van der Giessen, F. Zijlstra, and W. H. van Gilst. Rat abdominal aorta stenting: a new and reliable small animal model for in-stent restenosis. J. Vasc. Res. 41:377–386, 2004.
Lim, D., S. K. Cho, W. P. Park, A. Kristensson, J. Y. Ko, S. T. Al-Hassani, and H. S. Kim. Suggestion of potential stent design parameters to reduce restenosis risk driven by foreshortening or dogboning due to non-uniform balloon-stent expansion. Ann. Biomed. Eng. 36:1118–1129, 2008.
Malik, N., J. Gunn, C. Holt, L. Shepherd, S. Francis, C. Newman, D. Crossman, and D. Cumberland. Intravascular stents: a new technique for tissue processing for histology, immunohistochemistry and transmission electron microscopy. Heart 80:509–516, 1998.
Morlacchi, S., B. Keller, P. Arcangeli, M. Balzan, F. Migliavacca, G. Dubini, J. Gunn, N. Arnold, A. Narracott, D. Evans, and P. Lawford. Hemodynamics and in-stent restenosis: micro-CT images, histology, and computer simulations. Ann. Biomed. Eng. 39:2615–2626, 2011.
Onuma, Y., P. W. Serruys, L. E. Perkins, T. Okamura, N. Gonzalo, H. M. Garcia-Garcia, E. Regar, M. Kamberi, J. C. Powers, R. Rapoza, H. van Beusekom, W. van der Giessen, and R. Virmani. Intracoronary optical coherence tomography and histology at 1 month and 2, 3 and 4 years after implantation of everolimus-eluting bioresorbable vascular scaffolds in a porcine coronary artery model. Circulation 122:2288–2300, 2010.
Raina, T., J. Iqbal, N. Arnold, H. Moore, B. Aflatoonian, J. Walsh, S. Whitehouse, K. Al-Lamee, S. Francis, and J. Gunn. Coronary stents seeded with human trophoblastic endovascular progenitor cells show accelerated strut coverage without excessive neointimal proliferation in a porcine model. EuroIntervention 10:709–716, 2014.
Robinson, K. A., G. S. Roubin, R. J. Siegel, A. J. Black, R. P. Apkarian, and S. B. King. Intra-arterial stenting in the atherosclerotic rabbit. Circulation 78:646–653, 1988.
Rodriguez-Menocal, L., Y. Wei, S. M. Pham, M. St-Pierre, S. Li, K. Webster, P. Goldschmidt-Clermont, and R. I. Vazquez-Padron. A novel mouse model of in-stent restenosis. Atherosclerosis 209:359–366, 2010.
Rogers, C., and E. R. Edelman. Endovascular stent design dictates experimental restenosis and thrombosis. Circulation 91:2995–3001, 1995.
Rogers, C., D. Y. Tseng, J. C. Squire, and E. R. Edelman. Balloon-artery interactions during stent placement: a finite element analysis approach to pressure, compliance, and stent design as contributors to vascular injury. Circ. Res. 84:378–383, 1999.
Roubin, G. S., K. A. Robinson, S. B. King, C. Gianturco, A. J. Black, J. E. Brown, R. J. Siegel, and J. S. Douglas. Early and late results of intracoronary arterial stenting after coronary angioplasty in dogs. Circulation 76:891–897, 1987.
Schatz, R. A., J. C. Palmaz, F. O. Tio, F. Garcia, O. Garcia, and S. R. Reuter. Balloon-expandable intracoronary stents in the adult dog. Circulation 76:450–457, 1987.
Schulz, C., R. A. Herrmann, C. Beilharz, J. Pasquantonio, and E. Alt. Coronary stent symmetry and vascular injury determine experimental restenosis. Heart 83:462–467, 2000.
Schwartz, R. S., E. Edelman, R. Virmani, A. Carter, J. F. Granada, G. L. Kaluza, N. A. F. Chronos, K. A. Robinson, R. Waksman, J. Weinberger, G. J. Wilson, and R. L. Wilensky. Drug eluting stents in preclinical studies. Circ. Cardiovasc. Intervent. 1:143–153, 2008.
Schwartz, R. S., K. C. Huber, J. G. Murphy, W. D. Edwards, A. R. Camrud, R. E. Vlietstra, and D. R. Holmes. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J. Am. Coll. Cardiol. 19:267–274, 1992.
Sigwart, U., J. Puel, V. Mirkovitch, F. Joffre, and L. Kappenberger. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N. Engl. J. Med. 316:701–706, 1987.
Sousa, J. E., M. A. Costa, A. C. Abizaid, A. S. Abizaid, F. Feres, I. M. Pinto, A. C. Seixas, R. Staico, L. A. Mattos, A. G. Sousa, R. Falotico, J. Jaeger, J. J. Popma, and P. W. Serruys. Lack of neointimal proliferation after implantation of sirolimus-coated stents in human coronary arteries. Circulation 103:192–195, 2001.
Suzuki, T., G. Kopia, S. Hayashi, L. R. Bailey, G. Llanos, R. Wilensky, B. D. Klugherz, G. Papandreou, P. Narayan, M. B. Leon, A. C. Yeung, F. Tio, P. S. Tsao, R. Falotico, and A. J. Carter. Stent-based sirolimus therapy reduces neointimal formation in a porcine coronary model. Circulation 104:1188–1193, 2001.
Tearney, G. J., I. K. Jang, D. H. Kang, H. T. Aretz, S. L. Houser, T. J. Brady, K. Schlendorf, M. Shishkov, and B. E. Bouma. Porcine coronary imaging in vivo by optical coherence tomography. Acta Cardiol. 55:233–237, 2000.
Thorpe, P. E., W. J. Hunter, 3rd, X. X. Zhan, P. S. Dovgan, and D. K. Agrawal. A noninjury, diet-induced swine model of atherosclerosis for cardiovascular-interventional research. Angiology 47(9):849–857, 1996.
Timmins, L. H., M. W. Miller, F. J. Clubb, and J. E. Moore. Increased artery wall stress post-stenting leads to greater intimal thickening. Lab. Invest. 91:955–967, 2011.
Tominaga, R., H. E. Kambic, H. Emoto, H. Harasaki, C. Sutton, and J. Hollman. Effects of design geometry of intravascular endoprostheses on stenosis rate in normal rabbits. Am. Heart J. 123:21–28, 1992.
US Department of Health and Human Services Food and Drug Administration. Guidance for Industry: Coronary Drug-Eluting Stents - Nonclinical and Clinical Studies. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM072193.pdf. 2008.
Van der Giessen, W. J., P. W. Serruys, L. J. van Woerkens, K. J. Beatt, W. J. Visser, J. F. Jongkind, R. H. van Bremen, E. Ridderhof, H. van Loon, and L. K. Soei. Arterial stenting with self-expandable and balloon-expandable endoprostheses. Int J. Card. Imaging 5:163–171, 1990.
Van der Giessen, W. J., C. J. Slager, H. M. van Beusekom, D. S. van Ingen Schenau, R. A. Huijts, J. C. Schuurbiers, W. J. de Klein, P. W. Serruys, and P. D. Verdouw. Development of a polymer endovascular prosthesis and its implantation in porcine arteries. J. Interv. Cardiol. 5:175–185, 1992.
Van der Heiden, K., F. J. Gijssen, A. Narracott, S. Hsaio, I. Halliday, J. Gunn, J. J. Wentzel, and P. C. Evans. The effects of stenting on shear stress: relevance to endothelial injury and repair. Cardiovasc. Res. 99:269–275, 2013.
Vorpahl, M., J. R. Foerst, M. Kjelm, A. V. Kaplan, R. Virmani, and T. Ball. The complementary role of microCT and histopathology in characterizing the natural history of stented arteries. Expert Rev. Cardiovasc. Ther. 9:939–948, 2011.
Wentzel, J. J., F. J. Gijssen, N. Stergiopulos, P. W. Serruys, C. J. Slager, and R. Krams. Shear stress, vascular remodelling and neointimal formation. J. Biomech. 36:681–688, 2003.
Wentzel, J. J., D. M. Whelan, W. J. van der Giessen, H. M. van Beusekom, I. Andhyiswara, P. W. Serruys, C. J. Slager, and R. Krams. Coronary stent implantation changes 3-D vessel geometry and 3-D shear stress distribution. J. Biomech. 33:1287–1295, 2000.
Yoon, H. J., H. Y. Song, J. H. Kim, K. S. Hong, Y. J. Kim, H. G. Park, and D. K. Kim. Role of IN-1233 in the prevention of neointimal hyperplasia after stent placement in a rat artery model. J. Vasc. Interv. Radiol. 22:1321–1328, 2011.
Zarins, C. K., and C. A. Taylor. Endovascular device design in the future: transformation from trial and error to computational design. J. Endovasc. Ther. 16(1):I12–I21, 2009.
Acknowledgments
We are grateful to Nadine Arnold, for her veterinary and laboratory skills; and to the following for funding our animal work: British Heart Foundation, Department of Trade and Industry, Medical Research Council, Sheffield Hospitals Charitable Trust, Sir Jules Thorn Charitable Trust.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Peter E. McHugh oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Iqbal, J., Chamberlain, J., Francis, S.E. et al. Role of Animal Models in Coronary Stenting. Ann Biomed Eng 44, 453–465 (2016). https://doi.org/10.1007/s10439-015-1414-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-015-1414-4