Abstract
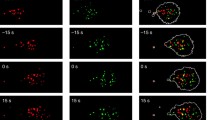
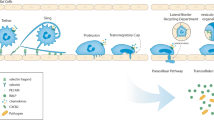
Neutrophils are key components of the immune system and motility is central their function during the inflammatory response. We have previously demonstrated that neutrophils are capable of switching their motile phenotype between amoeboid-like and keratocyte-like in response to the ligand density of adhesion molecules (Henry et al. in Int Biol 6:348–356, 2014). In this study, we engineered planar micropatterned surfaces that presented adhesion molecules in local islands of high density, separated by regions largely devoid of ligands. By controlling the geometry of islands we made arrays in which the local (on island) adhesion density was high but the global (multi-island) adhesion density over the entire cell-substrate interface was low. Neutrophils in contact with these island arrays assumed a well-spread and directionally-persistent motile phenotype (keratocyte-like) in contrast to the classical amoeboid morphology they display on uniform fields of high adhesion density. By virtue of our rationally designed substrates, we were able to conclude that neutrophils were integrating the stimulation received across their entire contact interface; furthermore, they were able to mount this whole cell response on the timescale of seconds. This work demonstrates the capacity of adhesive microenvironments to direct the phenotype of cell motility, which has broader implications in physiologic processes such as inflammation and cancer metastasis.






Similar content being viewed by others
References
Arnold, M., E. A. Cavalcanti-Adam, R. Glass, J. Blummel, W. Eck, M. Kantlehner, H. Kessler, and J. P. Spatz. Activation of integrin function by nanopatterned adhesive interfaces. Chemphyschem 5:383–388, 2004.
Barnhart, E. L., K. C. Lee, K. Keren, A. Mogilner, and J. A. Theriot. An adhesion-dependent switch between mechanisms that determine motile cell shape. PLoS Biol. 9:e1001059, 2011.
Borregaard, N. Neutrophils, from marrow to microbes. Immunity 33:657–670, 2010.
Cassimeris, L., H. McNeill, and S. H. Zigmond. Chemoattractant-stimulated polymorphonuclear leukocytes contain two populations of actin filaments that differ in their spatial distributions and relative stabilities. J. Cell Biol. 110:1067–1075, 1990.
Charras, G., and E. Sahai. Physical influences of the extracellular environment on cell migration. Nat. Rev. Mol. Cell Biol. 15:813–824, 2014.
Chen, C. S., J. L. Alonso, E. Ostuni, G. M. Whitesides, and D. E. Ingber. Cell shape provides global control of focal adhesion assembly. Biochem. Biophys. Res. Commun. 307:355–361, 2003.
Chen, C. S., M. Mrksich, S. Huang, G. M. Whitesides, and D. E. Ingber. Geometric control of cell life and death. Science 276:1425–1428, 1997.
Crocker, J. C., and B. D. Hoffman. Multiple-particle tracking and two-point microrheology in cells. Cell Mech. 83:141–178, 2007.
Desai, R., M. Yang, N. Sniadecki, W. Legant, and C. S. Chen. Microfabricated post-array-detectors (mPADs): an approach to isolate mechanical forces. J. Vis. Exp. 7:311, 2007.
Desai, R. A., M. K. Khan, S. B. Gopal, and C. S. Chen. Subcellular spatial segregation of integrin subtypes by patterned multicomponent surfaces. Integr. Biol. 3:560–567, 2011.
Dunn, G. A. Characterising a kinesis response: time averaged measures of cell speed and directional persistence. Agents Actions Suppl. 12:14–33, 1983.
Henry, S. J., J. C. Crocker, and D. A. Hammer. Ligand density elicits a phenotypic switch in human neutrophils. Integr. Biol. 6:348–356, 2014.
Jannat, R. A., G. P. Robbins, B. G. Ricart, M. Dembo, and D. A. Hammer. Neutrophil adhesion and chemotaxis depend on substrate mechanics. J. Phys.-Condens. Matter 22:194117, 2010.
Lammermann, T., B. L. Bader, S. J. Monkley, T. Worbs, R. Wedlich-Soldner, K. Hirsch, M. Keller, R. Forster, D. R. Critchley, R. Fassler, and M. Sixt. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453:51–55, 2008.
Lauffenburger D. A., and J. J. Linderman. Receptors: Models for Binding, Trafficking, and Signaling. New York: Oxford University Press, 1993, p. x, p. 365.
Lee, J., and K. Jacobson. The composition and dynamics of cell-substratum adhesions in locomoting fish keratocytes. J. Cell Sci. 110(Pt 22):2833–2844, 1997.
Lehnert, D., B. Wehrle-Haller, C. David, U. Weiland, C. Ballestrem, B. A. Imhof, and M. Bastmeyer. Cell behaviour on micropatterned substrata: limits of extracellular matrix geometry for spreading and adhesion. J. Cell Sci. 117:41–52, 2004.
Ley, K., C. Laudanna, M. I. Cybulsky, and S. Nourshargh. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immun. 7:678–689, 2007.
McDonald, B., K. Pittman, G. B. Menezes, S. A. Hirota, I. Slaba, C. C. M. Waterhouse, P. L. Beck, D. A. Muruve, and P. Kubes. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330:362–366, 2010.
Nathan, C. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6:173–182, 2006.
Oakes, P. W., D. C. Patel, N. A. Morin, D. P. Zitterbart, B. Fabry, J. S. Reichner, and J. X. Tang. Neutrophil morphology and migration are affected by substrate elasticity. Blood 114:1387–1395, 2009.
Paszek, M. J., N. Zahir, K. R. Johnson, J. N. Lakins, G. I. Rozenberg, A. Gefen, C. A. Reinhart-King, S. S. Margulies, M. Dembo, D. Boettiger, D. A. Hammer, and V. M. Weaver. Tensional homeostasis and the malignant phenotype. Cancer Cell 8:241–254, 2005.
Raptis, S. Z., S. D. Shapiro, P. M. Simmons, A. M. Cheng, and C. T. Pham. Serine protease cathepsin G regulates adhesion-dependent neutrophil effector functions by modulating integrin clustering. Immunity 22:679–691, 2005.
Smith, L. A., H. Aranda-Espinoza, J. B. Haun, M. Dembo, and D. A. Hammer. Neutrophil traction stresses are concentrated in the uropod during migration. Biophys. J. 92:L58–L60, 2007.
Stroka, K. M., and H. Aranda-Espinoza. Neutrophils display biphasic relationship between migration and substrate stiffness. Cell Motil Cytoskeleton 66:328–341, 2009.
Thiery, J. P., H. Acloque, R. Y. J. Huang, and M. A. Nieto. Epithelial-mesenchymal transitions in development and disease. Cell 139:871–890, 2009.
Vogel, V., and M. Sheetz. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 7:265–275, 2006.
Wang, N., E. Ostuni, G. M. Whitesides, and D. E. Ingber. Micropatterning tractional forces in living cells. Cell Motil Cytoskeleton 52:97–106, 2002.
Yang, M. T., J. Fu, Y. K. Wang, R. A. Desai, and C. S. Chen. Assaying stem cell mechanobiology on microfabricated elastomeric substrates with geometrically modulated rigidity. Nat. Protoc. 6:187–213, 2011.
Yao, D., C. Dai, and S. Peng. Mechanism of the mesenchymal-epithelial transition and its relationship with metastatic tumor formation. Mol. Cancer Res. 9:1608–1620, 2011.
Ziebert, F., and I. S. Aranson. Effects of adhesion dynamics and substrate compliance on the shape and motility of crawling cells. PLoS One 8:e64511, 2013.
Zigmond, S. H. Chemotaxis by polymorphonuclear leukocytes. J. Cell Biol. 77:269–287, 1978.
Acknowledgements
We are grateful to Eric Johnston for laboratory assistance and Christopher S. Chen, PhD and Ravi A. Desai, PhD for their time and expertise in teaching us the stamp-off method of microcontact printing. Funding for this work was provided by a National Science Foundation Graduate Research Fellowship to SJH and grants from the National Institutes of Health (HL18208 and GM104287) to DAH.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Konstantinos Konstantopoulos oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Movie S1
Amoeboid to keratocyte-like phenotypic switch. A small convective flow in the system pushes amoeboid neutrophils from the high density continuous field of fibronectin across the stamp-off control domain and into the hybrid islands domain. Rapid transitions on the timescale of seconds take place as amoeboid cells assume the keratocyte-like phenotype. No adhesion is observed in the stamp-off control domain implying that the residual protein between islands is not sufficiently stimulatory to support the keratocyte-like phenotype observed. Supplementary material 2 (MOV 882 kb)
Rights and permissions
About this article
Cite this article
Henry, S.J., Crocker, J.C. & Hammer, D.A. Motile Human Neutrophils Sense Ligand Density Over Their Entire Contact Area. Ann Biomed Eng 44, 886–894 (2016). https://doi.org/10.1007/s10439-015-1408-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-015-1408-2