Abstract
Patient-specific biomechanical properties of the human cornea are rarely used with finite elements analysis. In order for that to be possible, a proper formulation for biomechanical properties that is based on patient-specific measurable values must be used. In this study, we propose a formula that simulates hyperelastic stress–strain curves based on non-invasive clinical measurements that can be acquired in vivo. These consist of, but are not limited to, center corneal thickness and center corneal curvature as well as corneal resistance factor and applanation diameter that are measured during non-contact tonometry. The presented formulation was demonstrated and validated through several computer simulations. First, mean values that were reported in literature were inputted into the formula to simulate a curve that represents a healthy case. This case was compared to two independent in vitro studies. Then, a sensitivity analysis was carried to identify inputs that have the most dominant effect. Finally, a finite element analysis simulating elevations in intraocular pressure was conducted; the corneal model comprised of patient-specific corneal geometry that was measured in vivo in our clinic as well as the current formulation for patient-specific corneal biomechanics. “Strong” and “weak” corneal tissue cases were simulated and deformations as well as instantaneous curvature optical maps were derived. Results for the simulated healthy curve showed good agreement with the in vitro studies. The sensitivity analysis found the corneal resistance factor and applanation diameter to have the most dominant influence. The finite element analysis of strong and weak biomechanical properties resulted in corneal deformations and instantaneous curvature optical maps that are common for healthy and pathological conditions respectively. In conclusion, the presented modeling technique can be used to assess corneal biomechanics in vivo and therefor may enhance follow-up on the effectiveness of clinical treatments, rehabilitation of vision and perhaps improve the diagnosis of pathologies that are related to corneal biomechanics.








Similar content being viewed by others
References
Aghamohammadzadeh, H., R. H. Newton, and K. M. Meek. X-ray scattering used to map the preferred collagen orientation in the human cornea and limbus. Structure 12:249–256, 2004.
Ambrósio, Jr., R., I. Ramos, A. Luz, F. C. Faria, A. Steinmueller, M. Krug, M. W. Belin, and C. J. Roberts. Dynamic ultra high speed Scheimpflug imaging for assessing corneal biomechanical properties. Rev. Bras. Oftalmol. 72:99–102, 2013.
Aramberri, J., L. Araiz, A. Garcia, I. Illarramendi, J. Olmos, I. Oyanarte, A. Romay, and I. Vigara. Dual versus single Scheimpflug camera for anterior segment analysis: Precision and agreement. J. Cataract Refract. Surg. 38:1934–1949, 2012.
Asher, R., A. Gefen, and D. Varssano. In: Patient-Specific Modeling in Tomorrow’s Medicine, edited by A. Gefen. Berlin: Springer, 2012, Vol. 09, pp. 461–483.
Buzard, K. Introduction to biomechanics of the cornea. Refract. Corneal Surg. 8:127, 1992.
Chihara, E. Major review. Surv. Ophthalmol. 53:203–218, 2008.
Cook, J. A., A. P. Botello, A. Elders, A. Fathi Ali, A. Azuara-Blanco, C. Fraser, K. McCormack, and J. Margaret Burr. Systematic review of the agreement of tonometers with Goldmann applanation tonometry. Ophthalmology 119:1552–1557, 2012.
Deenadayalu, C., B. Mobasher, S. D. Rajan, and G. W. Hall. Refractive change induced by the LASIK flap in a biomechanical finite element model. J. Refract. Surg. 22:286, 2006.
Ehlers, N., T. Bramsen, and S. Sperling. Applanation tonometry and central corneal thickness. Acta Ophthalmol. Suppl. 53:34–43, 1975.
Elsheikh, A., D. Wang, and D. Pye. Determination of the modulus of elasticity of the human cornea. J. Refract. Surg. (Thorofare, NJ: 1995), 23:808, 2007.
Feltgen, N., D. Leifert, and J. Funk. Correlation between central corneal thickness, applanation tonometry, and direct intracameral IOP readings. Br. J. Ophthalmol. 85:85–87, 2001.
Fontes, B. M., R. Ambrósio, D. Jardim, G. C. Velarde, and W. Nosé. Corneal biomechanical metrics and anterior segment parameters in mild keratoconus. Ophthalmology 117:673–679, 2010.
Franco, S., and M. Lira. Biomechanical properties of the cornea measured by the Ocular Response Analyzer and their association with intraocular pressure and the central corneal curvature. Clin. Exp. Optom. 92:469–475, 2009.
Galletti, J., T. Pförtner, and F. Bonthoux. Improved keratoconus detection by ocular response analyzer testing after consideration of corneal thickness as a confounding factor. J. Refract. Surg. (Thorofare, NJ: 1995), 28:202–208, 2012.
Gasser, T. C., R. W. Ogden, and G. A. Holzapfel. Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J. R. Soc. Interface 3:15–35, 2006.
Gefen, A., R. Shalom, D. Elad, and Y. Mandel. Biomechanical analysis of the keratoconic cornea. J. Mech. Behav. Biomed. Mater. 2:224–236, 2009.
Grabner, G., R. Eilmsteiner, C. Steindl, J. Ruckhofer, R. Mattioli, and W. Husinsky. Dynamic corneal imaging. J. Cataract Refract. Surg. 31:163–174, 2005.
Grytz, R., and G. Meschke. A computational remodeling approach to predict the physiological architecture of the collagen fibril network in corneo-scleral shells. Biomech. Model. Mechanobiol. 9:225–235, 2010.
He, X., and J. Liu. A quantitative ultrasonic spectroscopy method for noninvasive determination of corneal biomechanical properties. Invest. Ophthalmol. Vis. Sci. 50:5148–5154, 2009.
Holzapfel, G. A., T. C. Gasser, and R. W. Ogden. A new constitutive framework for arterial wall mechanics and a comparative study of material models. J. Elast. 61:1–48, 2000.
Jordan, C. A., A. Zamri, C. Wheeldon, D. V. Patel, R. Johnson, and C. N. McGhee. Computerized corneal tomography and associated features in a large New Zealand keratoconic population. J. Cataract Refract. Surg. 37:1493–1501, 2011.
Karseras, A. G., and M. Ruben. Aetiology of keratoconus. Brit. J. Ophthalmol. 60:522–525, 1976.
Kling, S., L. Remon, A. Pérez-Escudero, J. Merayo-Lloves, and S. Marcos. Corneal biomechanical changes after collagen cross-linking from porcine eye inflation experiments. Invest. Ophthalmol. Vis. Sci. 51:3961–3968, 2010.
Kniestedt, C., O. Punjabi, S. Lin, and R. L. Stamper. Tonometry through the ages. Surv. Ophthalmol. 53:568–591, 2008.
Lau, W., and D. Pye. A clinical description of Ocular Response Analyzer measurements. Invest. Ophthalmol. Vis. Sci. 52:2911–2916, 2011.
Leonardi, M., P. Leuenberger, D. Bertrand, A. Bertsch, and P. Renaud. First steps toward noninvasive intraocular pressure monitoring with a sensing contact lens. Invest. Ophthalmol. Vis. Sci. 45:3113–3117, 2004.
Liu, J., and C. J. Roberts. Influence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysis. J. Cataract Refract. Surg. 31:146–155, 2005.
Luce, D. A. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J. Cataract Refract. Surg. 31:156–162, 2005.
Maas, S. A., B. J. Ellis, G. A. Ateshian, and J. A. Weiss. FEBio: finite elements for biomechanics. J. Biomech. Eng. 134, 2012.
Miháltz, K., I. Kovács, Á. Takács, and Z. Z. Nagy. Evaluation of keratometric, pachymetric, and elevation parameters of keratoconic corneas with pentacam. Cornea 28:976, 2009.
Orssengo, G. J., and D. C. Pye. Determination of the true intraocular pressure and modulus of elasticity of the human cornea in vivo. Bull. Math. Biol. 61:551–572, 1999.
Pandolfi, A., and F. Manganiello. A model for the human cornea: constitutive formulation and numerical analysis. Biomech. Model. Mechanobiol. 5:237–246, 2006.
Park, S.-H., S.-K. Choi, D. Lee, E.-J. Jun, and J.-H. Kim. Corneal thickness measurement using orbscan, pentacam, galilei, and ultrasound in normal and post-femtosecond laser in situ keratomileusis eyes. Cornea 31:978–982, 2012.
Pinsky, P. M., and D. V. Datye. A microstructurally-based finite element model of the incised human cornea. J. Biomech. 24:907–922, 1991.
Portnoy, S., N. Vuillerme, Y. Payan, and A. Gefen. Clinically oriented real-time monitoring of the individual’s risk for deep tissue injury. Med. Biol. Eng. Comput. 49:473–483, 2011.
Roy, A. S., and W. J. Dupps, Jr. Patient-specific modeling of corneal refractive surgery outcomes and inverse estimation of elastic property changes. J. Biomech. Eng. 133:011002, 2011.
Szalai, E., A. Berta, Z. Hassan, and L. Módis. Reliability and repeatability of swept-source Fourier-domain optical coherence tomography and Scheimpflug imaging in keratoconus. J. Cataract. Refract. Surg. 2012.
Uçakhan, Ö. Ö., V. Çetinkor, M. Özkan, and A. Kanpolat. Evaluation of Scheimpflug imaging parameters in subclinical keratoconus, keratoconus, and normal eyes. J. Cataract Refract. Surg. 37:1116–1124, 2011.
Varssano, D., R. Asher, and A. Gefen. Biomechanical modeling of the human eye with a focus on the cornea. In: Multi-Modality State-of-the-Art: Human Eye Imaging and Modeling, edited by E. Y. K. Ng. Boca Raton, FL: CRC Press. ISBN 9781439869932.
Wollensak, G., E. Spoerl, and T. Seiler. Stress-strain measurements of human and porcine corneas after riboflavin–ultraviolet-A-induced cross-linking. J. Cataract Refract. Surg. 29:1780–1785, 2003.
Woo, S.-Y., A. Kobayashi, W. Schlegel, and C. Lawrence. Nonlinear material properties of intact cornea and sclera. Exp. Eye Res. 14:29–39, 1972.
Yeter, V., B. Sönmez, and U. Beden. Comparison of central corneal thickness measurements by Galilei Dual-Scheimpflug analyzer® and ultrasound pachymeter in myopic eyes. Ophthalmic Surg. Lasers Imaging 43:128–134, 2012.
Young, W. C., and R. G. Budynas. Roark’s Formulas for Stress and Strain. New York: McGraw-Hill, 2002.
Zeng, Y., J. Yang, K. Huang, Z. Lee, and X. Lee. A comparison of biomechanical properties between human and porcine cornea. J. Biomech. 34:533–537, 2001.
Acknowledgments
This study was partially supported by a Grant from the Tel Aviv Sourasky Medical Center Research Foundation (A. G., D. V.).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Sigal Portnoy oversaw the review of this article.
Appendix
Appendix
Expansion of Eq. (4) to Include IOP0 (IOP at Time of Measurement)
Solving the differential Eq. (4) using non-homogenous boundary conditions results in:
Note that Eq. (13) becomes Eq. (5) by zeroing IOP0
Use Eq. (2) to give the residual stress for measured IOP0
An Explicit Definition of α and β
An Explicit Definition for A(μ)
A(μ) is a geometrical coefficient that was approximated using Taylor’s approximation as follows5,35:
(for 0 < μ < 1.4)
where \(\mu = \frac{AD}{2}\left[ {\frac{{12\left( {1 - \nu^{2} } \right)}}{{\left( {R - \frac{t}{2}} \right)^{2} \cdot t^{2} }}} \right]^{0.25}\) (AD is in our case the applanation diameter).
Limitations Imposed by A(μ)
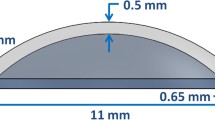
Special attention was given to the Taylor’s approximation, in order to clarify the constraints it imposes on the model. For that matter, a simulation was carried in order to show the dependency of \(A(\mu )\) on the range of possible inputs (CCT, CCR and AD). In each analysis (Figs. 9a, 9b and 9c) the horizontal dashed lines depict where the approximation limits are. Passing these margins will add a growing approximation error to the results. The CCT, CCR and AD were varied between 0.34–0.65 mm, 6–9 mm and 0.5–4 mm respectively. These ranges were chosen in order to cover a very wide of possible inputs, including very extreme pathological conditions.12–14,30,38
In each simulation the horizontal dashed lines depict where the approximation limits are. Passing these margins will add a growing error to the results. The CCT, CCR and applanation diameter were varied between 0.34–0.65 mm, 6–9 mm and 0.5–4 mm respectively. These ranges were chosen in order to cover a very wide of possible inputs, including very extreme pathological conditions12–14,30,38
The asterisk in Fig. 9 marks the values of the reference simulation (Fig. 2). Low CCT and CCR values move the asterisk towards lower A(μ) values. In contrast, low AD values move the asterisk upwards towards higher A(μ) values. The resulting lower limit for the CCT (Fig. 9a) is 0.4 mm and there is no upper limit. The CCR (Fig. 9b) has a lower limit of 6.1 mm and no upper limit as well. A(μ) is inside the boundaries for applanation diameters that are up to 3.5 mm and has no lower limit (Fig. 9c).
Rights and permissions
About this article
Cite this article
Asher, R., Gefen, A., Moisseiev, E. et al. An Analytical Approach to Corneal Mechanics for Determining Practical, Clinically-Meaningful Patient-Specific Tissue Mechanical Properties in the Rehabilitation of Vision. Ann Biomed Eng 43, 274–286 (2015). https://doi.org/10.1007/s10439-014-1147-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-014-1147-9