Abstract
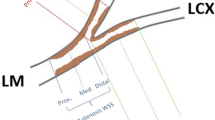
The purpose of the present study was to determine whether in vivo bifurcation geometric factors would permit prediction of the risk of atherosclerosis. It is worldwide accepted that low or oscillatory wall shear stress (WSS) is a robust hemodynamic factor in the development of atherosclerotic plaque and has a strong correlation with the local site of plaque deposition. However, it still remains unclear how coronary bifurcation geometries are correlated with such hemodynamic forces. Computational fluid dynamics simulations were performed on left main (LM) coronary bifurcation geometries derived from CT of eight patients without significant atherosclerosis. WSS amplitudes were accurately quantified at two high risk zones of atherosclerosis, namely at proximal left anterior descending artery (LAD) and at proximal left circumflex artery (LCx), and also at three high WSS concentration sites near the bifurcation. Statistical analysis was used to highlight relationships between WSS amplitudes calculated at these five zones of interest and various geometric factors. The tortuosity index of the LM-LAD segment appears to be an emergent geometric factor in determining the low WSS amplitude at proximal LAD. Strong correlations were found between the high WSS amplitudes calculated at the endothelial regions close to the flow divider. This study not only demonstrated that CT imaging studies of local risk factor for atherosclerosis could be clinically performed, but also showed that tortuosity of LM-LAD coronary branch could be used as a surrogate marker for the onset of atherosclerosis.






Similar content being viewed by others
Abbreviations
- ARLAD/LM :
-
Area ratio = LAD2 section area divided by LM2 section area
- ARLCx/LM :
-
Area ratio = LCx2 section area divided by LM2 section area
- CFD:
-
Computational fluid dynamics
- WSS:
-
Wall shear stress
- High-WSSLAD :
-
Highest WSS amplitude at proximal LAD (region #4, Fig. 5)
- High-WSSLCx :
-
Highest WSS amplitude at proximal LCx (region #3, Fig. 5)
- High-WSSLM :
-
Highest WSS amplitude at the LM site of interest (region #1, Fig. 5)
- LAD:
-
Left anterior descending artery
- LCx:
-
Left circumflex artery
- LM:
-
Left main artery
- Low-WSSLAD :
-
Lowest WSS amplitude at proximal LAD (region #5, Fig. 5)
- Low-WSSLCx :
-
Lowest WSS amplitude at proximal LCx (region #2, Fig. 5)
- P bif :
-
Coronary bifurcation point
- PLS:
-
Percentage luminal stenosis
- TortuosityLAD-LCx :
-
Tortuosity of the LAD + LCx arterial segment near the bifurcation
- TortuosityLM-LAD :
-
Tortuosity of the LM + LAD arterial segment near the bifurcation
- TortuosityLM-LCx :
-
Tortuosity of the LM + LCx arterial segment near the bifurcation
- \(\alpha _{\rm LM-LCx}\) :
-
Angle between LM and LCx branches (unit: °)
- \(\alpha _{\rm LM-LAD}\) :
-
Angle between LM and LAD branches (unit: °)
- α bif :
-
Bifurcation angle defined as the angle between LAD and LCx branches (unit: °)
References
Caro, C.G., J. M. Fitz-Gerald, and R. C. Schroter. Arterial wall shear and distribution of early atheroma in man. Nature 223:1159–1160, 1969.
Caro, C. G., J. M. Fitz-Gerald, and R. C. Schroter. Atheroma and arterial wall shear. Observation, correlation and proposal of a shear dependent mass transfer mechanism for atherogenesis. Proc. R. Soc. Lond. B Biol. Sci. 177:109–159, 1971.
Chaichana, T., Z. Sun, and J. Jewkes. Computation of hemodynamics in the left coronary artery with variable angulations. J. Biomech. 44:1869–1878, 2011.
Chatzizisis, Y. S., A. U. Coskun, M. Jonas, E. R. Edelman, C. L. Feldman, and P. H. Stone. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J. Am. Coll. Cardiol. 49:2379–2393, 2007.
Cheng, C., D. Tempel, R. van Haperen, et al. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 113:2744–2753, 2006.
Cheruvu, P. K., A. V. Finn, C. Gardner, et al. Frequency and distribution of thin-cap fibroatheroma and ruptured plaques in human coronary arteries: a pathologic study. J. Am. Coll. Cardiol. 50:940–949, 2007.
Del Corso, L., D. Moruzzo, B. Conte, M. Agelli, A. M. Romanelli, F. Pastine, M. Protti, F. Pentimone, and G. Baggiani. Tortuosity, kinking, and coiling of the carotid artery: expression of atherosclerosis or aging? Angiology 49:361–371, 1998.
Ethier, C. R., S. Prakash, D. A. Steinman, R. L. Leask, G. G. Couch, and M. Ojha. Steady flow separation patterns in a 45 degree junction. J. Fluid Mech. 411:1–38, 2000.
Fayad, Z. A., V. Fuster, K. Nikolaou, and C. Becker. Computed tomography and magnetic resonance imaging for noninvasive coronary angiography and plaque imaging: current and potential future concepts. Circulation 106:2026–2034, 2002.
Fry, D. L. Acute vascular endothelial changes associated with increased blood velocity gradients. Circ. Res. 22:165–197, 1968.
Gijsen, F. J., J. J. Wentzel, A. Thury, et al. A new imaging technique to study 3-D plaque and shear stress distribution in human coronary artery bifurcations in vivo. J. Biomech. 40:2349–2357, 2007.
Gijsen, F. J., J. J. Wentzel, A. Thury, et al. Strain distribution over plaques in human coronary arteries relates to shear stress. Am. J. Physiol. Heart Circ. Physiol. 295:H1608–H1614, 2008.
Glagov, S., E. Weisenberg, C. K. Zarins, R. Stankunavicius, and G. J. Kolettis. Compensatory enlargement of human atherosclerotic coronary arteries. N. Engl. J. Med. 316:1371–1375, 1987.
Goubergrits, L., U. Kertzscher, B. Schoneberg, E. Wellnhofer, C. Petz, and H. C. Hege. CFD analysis in an anatomically realistic coronary artery model based on non-invasive 3D imaging: comparison of magnetic resonance imaging with computed tomography. Int. J. Cardiovasc. Imaging 24:411–421, 2008.
Han, H. C. Twisted blood vessels: symptoms, etiology and biomechanical mechanisms. J. Vasc. Res. 49:185–187, 2012.
Hiroki, M., K. Miyashita, and M. Oda. Tortuosity of the white matter medullary arterioles is related to the severity of hypertension. Cerebrovasc. Dis. 13:242–250, 2002.
Huo, Y,, T. Wischgoll, and G. S. Kassab. Flow patterns in three-dimensional porcine epicardial coronary arterial tree. Am. J. Physiol. Heart Circ. Physiol. 293:H2959–H2970, 2007.
Jakob, M., D. Spasojevic, O. N. Krogmann, H. Wiher, R. Hug, and O. M. Hess. Tortuosity of coronary arteries in chronic pressure and volume overload. Cathet. Cardiovasc. Diagn. 38:25–31, 1996.
Johnson, K., P. Sharma, and J. Oshinski. Coronary artery flow measurement using navigator echo gated phase contrast magnetic resonance velocity mapping at 3.0 T. J. Biomech. 41:595–602, 2008.
Johnston, B. M., P. R. Johnston, S. Corney, and D. Kilpatrick. Non-Newtonian blood flow in human right coronary arteries: steady state simulations. J. Biomech. 37:709–720, 2004.
Johnston BM, Johnston PR, Corney S, and Kilpatrick D. Non-Newtonian blood flow in human right coronary arteries: transient simulations. J. Biomech. 39:1116–1128, 2006.
Joshi, A. K., R. L. Leask, J. G. Myers, M. Ojha, J. Butany, and C. R. Ethier. Intimal thickness is not associated with wall shear stress patterns in the human right coronary artery. Arterioscler. Thromb. Vasc. Biol. 24:2408–2413, 2004.
Katouzian, A., S. Sathyanarayana, B. Baseri, E. E. Konofagou, and S. G. Carlier. Challenges in atherosclerotic plaque characterization with intravascular ultrasound (IVUS): from data collection to classification. IEEE Trans. Inf. Technol. Biomed. 12:315–327, 2008.
Kotys, M. S., D. A. Herzka, E. J. Vonken, et al. Profile order and time-dependent artifacts in contrast-enhanced coronary MR angiography at 3T: origin and prevention. Magn. Reson. Med. 62:292–299, 2009.
Kubo, T., T. Imanishi, S. Takarada, et al. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J. Am. Coll. Cardiol. 50:933–939, 2007.
Lee, S. W., L. Antiga, J. D. Spence, and D. A. Steinman. Geometry of the carotid bifurcation predicts its exposure to disturbed flow. Stroke 39:2341–2347, 2008.
Lehoux, S., Y. Castier, and A. Tedgui. Molecular mechanisms of the vascular responses to haemodynamic forces. J. Intern. Med. 259:381–392, 2006.
MacLean, N. F., and M. R. Roach. Thickness, taper, and ellipticity in the aortoiliac bifurcation of patients aged 1 day to 76 years. Heart Vessels 13:95–101, 1998.
Malek, A. M., S. L. Alper, and S. Izumo. Hemodynamic shear stress and its role in atherosclerosis. JAMA 282:2035–2042, 1999.
Medina, A., J. Suarez de Lezo, and M. Pan. A new classification of coronary bifurcation lesions. Rev. Esp. Cardiol. 59(2):183–184, 2006.
Murray, C. D. The Physiological principle of minimum work: I. The vascular system and the cost of blood volume. Proc. Natl Acad. Sci. U.S.A. 12:207–214, 1926.
Nissen, S. E., J. C. Gurley, C. L. Grines, D. C. Booth, R. McClure, M. Berk, C. Fischer, and A. N. DeMaria. Intravascular ultrasound assessment of lumen size and wall morphology in normal subjects and patients with coronary artery disease. Circulation 84(3):1087–99, 1991.
Ong, C. W., S. Dokos, B. T. Chan, E. Lim, A. Al Abed, N. A. B. Abu Osman, S. Kadiman, N. H. Lovell. Numerical investigation of the effect of cannula placement on thrombosis. Theor. Biol. Med. Model. 2013:10–35, 2013.
Pancera, P., M. Ribul, B. Presciuttini, A. Lechi. Prevalence of carotid artery kinking in 590 consecutive subjects evaluated by echo-color Doppler. Is there a correlation with arterial hypertension? J. Intern. Med. 248:7–12, 2000.
Rioufol, G., M. Gilard, G. Finet, I. Ginon, J. Boschat, and X. Andre-Fouet. Evolution of spontaneous atherosclerotic plaque rupture with medical therapy: long-term follow-up with intravascular ultrasound. Circulation 110:2875–2880, 2004.
Sankaranarayanan, M., L. P. Chua, D. N. Ghista, and Tan, Y. S. Computational model of blood flow in the aorto-coronary bypass graft. Biomed. Online 4:1–14, 2005.
Sanz, J., and Z. A. Fayad. Imaging of atherosclerotic cardiovascular disease. Nature 451:953–957, 2008.
Soulis, J. V., T. M. Farmakis, G. D. Giannoglou, and G. E. Louridas. Wall shear stress in normal left coronary artery tree. J. Biomech. 39:742–749, 2006.
Stone, P. H., A. U. Coskun, S. Kinlay, et al. Effect of endothelial shear stress on the progression of coronary artery disease, vascular remodeling, and in-stent restenosis in humans: in vivo 6-month follow-up study. Circulation 108:438–444, 2003.
Tearney, G. J., S. Waxman, M. Shishkov, et al. Three-dimensional coronary artery microscopy by intracoronary optical frequency domain imaging. JACC Cardiovasc. Imaging 1:752–761, 2008.
Thomas, J. B., L. Antiga, S. L. Che, et al. Variation in the carotid bifurcation geometry of young versus older adults: implications for geometric risk of atherosclerosis. Stroke 36:2450–2456, 2005.
Thomas, J. B., J. S. Milner, and D. A. Steinman. On the influence of vessel planarity on local hemodynamics at the human carotid bifurcation. Biorheology 39:443–448, 2002.
van Wyk, S., L. Prahl Wittberg, and L. Fuchs. Wall shear stress variations and unsteadiness of pulsatile blood-like flows in 90-degree bifurcations. Comput. Biol. Med. 43:1025–1036, 2013.
van Wyk, S., L. Prahl Wittberg, and L. Fuchs. Atherosclerotic indicators for blood-like fluids in 90-degree arterial-like bifurcations. Comput. Biol. Med. 50:56–69, 2014.
Weibel, J., and W. S. Fields. Tortuosity, coiling, and kinking of the internal carotid artery. I. Etiology and radiographic anatomy. Neurology 15:7–18, 1965.
Wellnhofer, E., L. Goubergrits, U. Kertzscher, K. Affeld, and E. Fleck. Novel non-dimensional approach to comparison of wall shear stress distributions in coronary arteries of different groups of patients. Atherosclerosis 202:483–490, 2009.
Wellnhofer, E., J., U. Kertzscher, K. Affeld, E. Fleck, and L. Goubergrits. Non-dimensional modeling in flow simulation studies of coronary arteries including side-branches: a novel diagnostic tool in coronary artery disease. Atherosclerosis 216:277–282, 2011.
Zakaria, H., A. M. Robertson, and C. W. Kerber. A parametric model for studies of flow in arterial bifurcations. Ann. Biomed. Eng. 36:1515–1530, 2008.
Zeng, D., Z. Ding, M. H. Friedman, and C. R. Ethier. Effects of cardiac motion on right coronary artery hemodynamics. Ann. Biomed. Eng. 31:420–429, 2003.
Zhu, H., Z. Ding, R. N. Piana, T. R. Gehrig, and M. H. Friedman. Cataloguing the geometry of the human coronary arteries: a potential tool for predicting risk of coronary artery disease. Int. J. Cardiol. 135:43–52, 2009.
Acknowledgments
M. Malvè and M.A. Martínez are supported by the Spanish Ministry of Science and Technology through research project DPI-2010-20746-C03-01 and the Instituto de Salud Carlos III (ISCIII) through the CIBER-BBN initiative. This research was supported in part by an appointment (J. Ohayon) to the Senior Fellow Program at the National Institutes of Health (NIH). This program is administered by Oak Ridge Institute for Science and Education through an interagency agreement between the NIH and the U.S. Department of Energy.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Associate Editor Umberto Morbiducci oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Malvè, M., Gharib, A.M., Yazdani, S.K. et al. Tortuosity of Coronary Bifurcation as a Potential Local Risk Factor for Atherosclerosis: CFD Steady State Study Based on In Vivo Dynamic CT Measurements. Ann Biomed Eng 43, 82–93 (2015). https://doi.org/10.1007/s10439-014-1056-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-014-1056-y