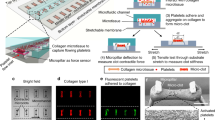
Abstract
The high blood volume requirements and low throughput of conventional flow assays for measuring platelet function are unsuitable for drug screening and clinical applications. In this study, we describe a microfluidic flow assay that uses 50 μL of whole blood to measure platelet function on ~300 micropatterned spots of collagen over a range of physiologic shear rates (50–920 s−1). Patterning of collagen thin films (CTF) was achieved using a novel hydrated microcontact stamping method. CTF spots of 20, 50, and 100 μm were defined on glass substrates and consisted of a dense mat of nanoscale collagen fibers (3.74 ± 0.75 nm). We found that a spot size of greater than 20 μm was necessary to support platelet adhesion under flow, suggesting a threshold injury size is necessary for stable platelet adhesion. Integrating 50 μm CTF microspots into a multishear microfluidic device yielded a high content assay from which we extracted platelet accumulation metrics (lag time, growth rate, total accumulation) on the spots using Hoffman modulation contrast microscopy. This method has potential broad application in identifying platelet function defects and screening, monitoring, and dosing antiplatelet agents.








Similar content being viewed by others
References
Basabe-Desmonts, L., S. Ramstrom, G. Meade, S. O’neill, A. Riaz, L. P. Lee, A. J. Ricco, and D. Kenny. Single-step separation of platelets from whole blood coupled with digital quantification by interfacial platelet cytometry (iPC). Langmuir 26:14700–14706, 2010. doi:10.1021/la9039682.
Bernard, A., J. Renault, B. Michel, H. Bosshard, and E. Delamarche. Microcontact printing of proteins. Adv. Mater. 12:1067–1070, 2000.
Bessueille, F., M. Pla-Roca, C. A. Mills, E. Martinez, J. Samitier, and A. Errachid. Submerged microcontact printing (SμCP): an unconventional printing technique of thiols using high aspect ratio, elastomeric stamps. Langmuir 21:12060–12063, 2005.
Bickle, M. The beautiful cell: high-content screening in drug discovery. Anal. Bioanal. Chem. 398:219–226, 2010.
Cheong, R., S. Paliwal, and A. Levchenko. High-content screening in microfluidic devices. Expert Opin. Drug Discov. 5:715–720, 2010.
Conant, C. G., M. A. Schwartz, J. E. Beecher, R. C. Rudoff, C. Ionescu-Zanetti, and J. T. Nevill. Well plate microfluidic system for investigation of dynamic platelet behavior under variable shear loads. Biotechnol. Bioeng. 108:2978–2987, 2011.
Farndale, R. W., J. J. Sixma, M. J. Barnes, and P. G. de Groot. The role of collagen in thrombosis and hemostasis. J. Thromb. Haemost. 2:561–573, 2004.
Fukuda, J., Y. Sakai, and K. Nakazawa. Novel hepatocyte culture system developed using microfabrication and collagen/polyethylene glycol microcontact printing. Biomaterials 27:1061–1070, 2006.
Gurdak, E., P. G. Rouxhet, and C. C. Dupont-Gillain. Factors and mechanisms determining the formation of fibrillar collagen structures in adsorbed phases. Colloids Surf. B 52:76–88, 2006.
Gutierrez, E., B. G. Petrich, S. J. Shattil, M. H. Ginsberg, A. Groisman, and A. Kasirer-Friede. Microfluidic devices for studies of shear-dependent platelet adhesion. Lab Chip 8:1486–1495, 2008.
Hansen, R. R., A. A. Tipnis, T. C. White-Adams, J. A. Di Paola, and K. B. Neeves. Characterization of collagen thin films for von Willebrand factor binding and platelet adhesion. Langmuir 27:13648–13658, 2011.
Hoffman, R., and L. Gross. Modulation contrast microscope. Appl. Opt. 14:1169–1176, 1975.
Hui, C., A. Jagota, Y. Lin, and E. Kramer. Constraints on microcontact printing imposed by stamp deformation. Langmuir 18:1394–1407, 2002.
Li, M., D. N. Ku, and C. R. Forest. Microfluidic system for simultaneous optical measurement of platelet aggregation at multiple shear rates in whole blood. Lab Chip 12:1355, 2012.
Nalayanda, D. D., M. Kalukanimuttam, and D. W. Schmidtke. Micropatterned surfaces for controlling cell adhesion and rolling under flow. Biomed. Microdevices 9:207–214, 2007.
Neeves, K. B., D. A. R. Illing, and S. L. Diamond. Thrombin flux and wall shear rate regulate fibrin fiber deposition state during polymerization under flow. Biophys. J. 98:1344–1352, 2010.
Neeves, K. B., S. F. Maloney, K. P. Fong, A. A. Schmaier, M. L. Kahn, L. F. Brass, and S. L. Diamond. Microfluidic focal thrombosis model for measuring murine platelet deposition and stability: PAR4 signaling enhances shear-resistance of platelet aggregates. J. Thromb. Haemost. 6:2193–2201, 2008.
Nemerson, Y., and V. Turitto. The effect of flow on hemostasis and thrombosis. Thromb. Haemost. 66:272–276, 1991.
Okorie, U. M., and S. L. Diamond. Matrix protein microarrays for spatially and compositionally controlled microspot thrombosis under laminar flow. Biophys. J. 91:3474–3481, 2006.
Orth, R., M. Wu, D. Holowka, H. Craighead, and B. Baird. Mast cell activation on patterned lipid bilayers of subcellular dimensions. Langmuir 19:1599–1605, 2003.
Philipose, S., V. Konya, I. Sreckovic, G. Marsche, I. Lippe, B. Peskar, A. Heinemann, and R. Schuligoi. The prostaglandin E2 receptor EP4 is expressed by human platelets and potently inhibits platelet aggregation and thrombus formation. Arterioscler. Thromb. Vasc. Biol. 30:2416, 2010.
Prabhakarpandian, B., M.-C. Shen, K. Pant, and M. F. Kiani. Microfluidic devices for modeling cell–cell and particle–cell interactions in the microvasculature. Microvasc. Res. 82:210–220, 2011.
Rozkiewicz, D. I., Y. Kraan, M. W. T. Werten, F. A. de Wolf, V. Subramaniam, B. J. Ravoo, and D. N. Reinhoudt. Covalent microcontact printing of proteins for cell patterning. Chem. Eur. J. 12:6290–6297, 2006.
Ruggeri, Z. M., and G. L. Mendolicchio. Adhesion mechanisms in platelet function. Circ. Res. 100:1673–1685, 2007.
Runyon, M. K., B. L. Johnson-Kerner, C. J. Kastrup, T. G. Van Ha, and R. F. Ismagilov. Propagation of blood clotting in the complex biochemical network of hemostasis is described by a simple mechanism. J. Am. Chem. Soc. 129:7014–7015, 2007.
Sarratt, K. L., H. Chen, M. M. Zutter, S. A. Santoro, D. A. Hammer, and M. L. Kahn. GPVI and alpha2beta1 play independent critical roles during platelet adhesion and aggregate formation to collagen under flow. Blood 106:1268–1277, 2005.
Sarvepalli, D. P., D. W. Schmidtke, and M. U. Nollert. Design considerations for a microfluidic device to quantify the platelet adhesion to collagen at physiological shear rates. Ann. Biomed. Eng. 37:1331–1341, 2009.
Savage, B., M. H. Ginsberg, and Z. M. Ruggeri. Influence of fibrillar collagen structure on the mechanisms of platelet thrombus formation under flow. Blood 94:2704–2715, 1999.
Shen, F., C. J. Kastrup, Y. Liu, and R. F. Ismagilov. Threshold response of initiation of blood coagulation by tissue factor in patterned microfluidic capillaries is controlled by shear rate. Arterioscler. Thromb. Vasc. Biol. 28:2035–2041, 2008.
Tovar-Lopez, F. J., G. Rosengarten, E. Westein, K. Khoshmanesh, S. P. Jackson, A. Mitchell, and W. S. Nesbitt. A microfluidics device to monitor platelet aggregation dynamics in response to strain rate micro-gradients in flowing blood. Lab Chip 10:291, 2010.
Yen, R. T., and Y. C. Fung. Effect of velocity of distribution on red cell distribution in capillary blood vessels. Am. J. Physiol. 235:H251–H257, 1978.
Acknowledgments
This work was supported by a Scientist Development Grant (K.B.N.) and a Postdoctoral Fellowship (R.R.H) from the American Heart Association, the National Heart, Lung, and Blood Institute (HL100333), the Colorado Office of Economic Development and International Trade, and the Boettcher Foundation’s Webb-Waring Biomedical Research Award.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael R. King oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material includes figures of the μCP apparatus, image processing routine, immunofluorescent images of micropatterned CTF, and the layout and characterization of the msMFA. Supplementary Movies 1, 2, and 3 show platelet accumulation on 20, 50, and 100 μm CTF spots, respectively, using HMC microscopy. The colored circles represent the location of the CTF spots during whole blood flow assay. Supplementary Movie 4 shows the relative fluid velocity via fluorescence polystyrene beads in the six channels of the msMFA. Supplementary Movies 5, 6, and 7 show platelet accumulation in the msMFA on 50 μm CTP spots for donors 2, 3 and 4, respectively.
Supplementary material 1 (MOV 16484 kb)
Supplementary material 2 (MOV 13577 kb)
Supplementary material 3 (MOV 16761 kb)
Supplementary material 4 (MOV 4199 kb)
Supplementary material 5 (MOV 3362 kb)
Supplementary material 6 (MOV 3499 kb)
Supplementary material 7 (MOV 5191 kb)
Rights and permissions
About this article
Cite this article
Hansen, R.R., Wufsus, A.R., Barton, S.T. et al. High Content Evaluation of Shear Dependent Platelet Function in a Microfluidic Flow Assay. Ann Biomed Eng 41, 250–262 (2013). https://doi.org/10.1007/s10439-012-0658-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-012-0658-5