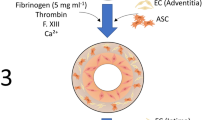
Mechanical conditioning represents a potential means to enhance the biochemical and biomechanical properties of tissue engineered vascular grafts (TEVGs). A pulsatile flow bioreactor was developed to allow shear and pulsatile stimulation of TEVGs. Physiological 120 mmHg/80 mmHg peak-to-trough pressure waveforms can be produced at both fetal and adult heart rates. Flow rates of 2 mL/sec, representative of flow through small diameter blood vessels, can be generated, resulting in a mean wall shear stress of ∼6 dynes/cm2 within the 3 mm ID constructs. When combined with non-thrombogenic poly(ethylene glycol) (PEG)-based hydrogels, which have tunable mechanical properties and tailorable biofunctionality, the bioreactor represents a flexible platform for exploring the impact of controlled biochemical and biomechanical stimuli on vascular graft cells. In the present study, the utility of this combined approach for improving TEVG outcome was investigated by encapsulating 10T-1/2 mouse smooth muscle progenitor cells within PEG-based hydrogels containing an adhesive ligand (RGDS) and a collagenase degradable sequence (LGPA). Constructs subjected to 7 weeks of biomechanical conditioning had significantly higher collagen levels and improved moduli relative to those grown under static conditions.




Similar content being viewed by others
REFERENCES
Anseth, K. S., C. N. Bowman, and L. BrannonPeppas. Mechanical properties of hydrogels and their experimental determination. Biomaterials 17:1647–1657, 1996.
Birukov, K., and V. Shirinsky. Stretch affects phenotype and proliferation of vascular smooth muscle cells. Mol. Cell Biochem. 144:131–139, 1995.
Brossollet, L. Mechanical issues in vascular grafting: a review. Int. J. Artif. Organs 15:579–584, 1992.
Bryant, S. B., R. J. Durand, and K. Anseth. Encapsulating chondrocytes in degrading PEG hydrogels with high modulus: Engineering gel structural changes to facilitate cartilaginous tissue production. Biotechnol. Bioeng. 86:747–755, 2004.
Bryant, S. J., and K. S. Anseth. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J. Biomed. Mater. Res. 59:63–72, 2002.
Bryant, S. J., C. R. Nuttelman, and K. S. Anseth. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J. Biomater. Sci. Polym. Ed. 11:439–457, 2000.
Bryant, S., K. Anseth, D. Lee, and D. Bader. Crosslinking density influences the morphology of chondrocytes photoencapsulated in PEG hydrogels during the application of compressive strain. J. Orthop. Res. 22:1143–1149, 2004.
Bryant, S., T. Chowdhury, D. Lee, D. Bader, and K. Anseth. Crosslinking density influences chondrocyte metabolism in dynamically loaded photocrosslinked poly(ethylene glycol) hydrogels. Ann. Biomed. Eng. 32:407–417, 2004.
Burdick, J., and K. Anseth. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials 23:4315–4323, 2002.
Cappadona, C. et al. Phenotype dictates the growth response of vascular smooth muscle cells to pulse pressure in vitro. Exp. Cell Res. 250:174–186, 1999.
Cheng, G., and W. Briggs. Mechanical strain tightly controls fibroblast growth factor-2 release from cultured human vascular smooth muscle cells. Circ. Res. 80:28–36, 1997.
Chiquet, M., and M. Matthisson. Regulation of extracellular matrix synthesis by mechanical stress. Biochem. Cell Biol. 74:737–744, 1996.
Clerin, V. et al. Tissue engineering of arteries by directed remodeling of intact arterial segments. Tissue Engineering 9: 2003.
Elisseeff, J. et al. Transdermal photopolymerization for minimally invasive implantation. Proc. Nat. Acad. Sci. U.S.A. 96:3104–3107, 1999.
Faries, P. et al. A comparative study of alternative conduits for lower extremity revascularization: all-autogenous conduit versus prosthetic grafts. J. Vasc. Surg. 32:1080–1090, 2000.
Fung, Y. C. Biomechanics: Mechanical Properties of Living Tissues. New York: Springer-Verlag, 1993.
Gobin, A. S., and J. L. West. Cell migration through defined, synthetic extracellular matrix analogues. FASEB J. 16:2002.
Gombotz, W. R., G. H. Wang, T. A. Horbett, and A. S. Hoffman. Protein adsorption to poly(ethylene oxide) surfaces. J. Biomed. Mater. Res. 25:1547–1562, 1991.
Gomes, M. E., V. I. Sikavitsas, E. Behravesh, R. L. Reis, and A. G. Mikos. Effect of flow perfusion on the osteogenic differentiation of bone marrow stromal cells cultured on starch-based three-dimensional scaffolds. J. Biomed. Mater. Res. A. 67:87–95, 2003.
Gregory, T. R. Nucleotypic effects without nuclei: Genome size and erythrocyte size in mammals. Genome 43:895–901, 2000.
Greisler, H. Interactions at the blood/material interface. Ann. Vasc. Surg. 4:98–103, 1990.
Hiles, M. et al. Mechanical properties of xenogeneic small-intestinal submucosa when used as an aortic graft in the dog. J. Biomed. Mater. Res. 29:883–891, 1995.
Hill-West, J. L., S. M. Chowdhury, M. J. Slepian, and J. A. Hubbell. Inhibition of thrombosis and intimal thickening by in situ photopolymerization of thin hydrogel barriers. Proc. Nat. Acad. Sci. U.S.A. 91:5967–5971, 1994.
Hirschi, K. K., J. M. Burt, K. D. Hirschi, and C. Dai. Gap junction communication mediates TGF-β activation and endothelial-induced mural cell differentiation. Circ. Res. 93:429–437, 2003.
Isenberg, B., and R. Tranquillo. Long-term cyclic distention enhances the mechanical properties of collagen-based media-equivalents. Ann. Biomed. Eng. 31:937–939, 2003.
Jeong, S. I. et al. Mechano-active tissue engineering of vascular smooth muscle using pulsatile perfusion bioreactors and elastic PLCL scaffolds. Biomaterials 26:1405–1411, 2005.
Johnson, C., T. How, M. Scraggs, C. West, and J. Burns. A biomechanical study of the human vertebral artery with implications for fatal arterial injury. Forensic Sci. Int. 109:169–182, 2000.
Kanda, K., and T. Matsuda. Mechanical stress induced cellular orientation and phenotypic modulation of 3D cultured smooth muscle cells. ASAIO 39: 1993.
Kempczinski, R. (ed.) Vascular Surgery. Denver: WB Saunders, 2000.
Kim, B. S., J. Nikolovski, J. Bonadio, and D. Mooney. Cyclic mechanical strain regulates the development of engineered smooth muscle cell tissue. Nat. Biotechnol. 17:979–983, 1999.
Ku, D., and C. Zhu. The mechanical environment of the artery. In: Hemodynamic Forces and Vascular Cell Biology, edited by B. Sumpio. Austin: RG Landes Company, 1993, pp. 1–23.
Kulik, T., and S. Alvarado. Effect of stretch on growth and collagen synthesis in cultured rat and lamb pulmonary arterial smooth muscle cells. J. Cell. Physiol. 157:615–624, 1993.
Li, C., and Q. Xu. Mechanical stress-initiated signal transductions in vascular smooth muscle cells. Cell. Signaling 12:435–445, 2000.
Liu, V. A., and S. N. Bhatia. Three-dimensional photopatterning of hydrogels containing living cells. Biomed. Microdevices 4:257–266, 2002.
Long, J., and R. Tranquillo. Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biol. 22:339–350, 2003.
Lu, H. H., M. D. Kofron, S. F. El-Amin, M. A. Attawia, and C. T. Laurencin. In vitro bone formation using muscle-derived cells: a new paradigm for bone tissue engineering using polymer-bone morphogenetic protein matrices. Biochem. Biophys. Res. Commun. 305:882–889, 2003.
Miller, E. J., and S. Gay. Collagen – an Overview. Methods Enzymol. 82:3–32, 1982.
Niklason, L. et al. Functional arteries grown in vitro. Science 284:489–493, 1999.
Posey, J., and L. Geddes. Measurement of the modulus of elasticity of the arterial wall. Cardiovasc. Res. Ctr. Bull. 11:83–88, 1973.
Ross, J., and R. Tranquillo. ECM gene expression correlates with in vitro tissue growth and development in fibrin gel remodeled by neonatal smooth muscle cells. Matrix Biol. 22:477–490, 2003.
Schmedlen, R. H., W. M. Elbjeirami, Gobin, A. S., and J. L. West. Tissue engineered small-diameter vascular grafts. Clin. Plast. Surg. 30:507-+, 2003.
Solan, A., S. Mitchell, M. Moses, and L. E. Niklason. Effect of pulse rate on collagen deposition in the tissue-engineered blood vessel. Tissue Eng. 9:579–586, 2003.
Tranquillo, R., T. Girton, B. Bromberek, T. Triebes, and D. Mooradian. Magnetically orientated tissue-equivalent tubes: application to a circumferentially orientated media-equivalent. Biomaterials 17:349–357, 1996.
Vacanti, J., and R. Langer. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 354:SI32–SI34, 1999.
West, J. L., and J. A. Hubbell. Photopolymerized hydrogel materials for drug delivery applications. Reactive Polym. 25:139–147, 1995.
Whittemore, A., K. Kent, M. Donaldson, N. Couch, and J. Mannick. What is the proper role of polytetrafluoroethylene grafts in infrainguinal reconstruction? J. Vasc. Surg. 10:299–305, 1989.
Williams, C. G., A. N. Malik, T. K. Kim, P. N. Manson, and Jh, E. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials 26:1211–1218, 2005.
Wilson, E. K. Sudhir, and H. Ives. Mechanical strain of rat vascular smooth muscle cells is sensed by specific extracellular matrix/integrin interactions. J. Clin. Invest. 96:2364–2372, 1995.
ACKNOWLEDGMENTS
The authors would like to acknowledge funding from the NIH and NSF and a Whitaker Foundation Graduate Research Fellowship to MKM. We thank Jane Grande-Allen, PhD for advice regarding mechanical testing and biochemical analyses, and Marcella Estrella for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work
Rights and permissions
About this article
Cite this article
Hahn, M.S., McHale, M.K., Wang, E. et al. Physiologic Pulsatile Flow Bioreactor Conditioning of Poly(ethylene glycol)-based Tissue Engineered Vascular Grafts. Ann Biomed Eng 35, 190–200 (2007). https://doi.org/10.1007/s10439-006-9099-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-006-9099-3