Abstract
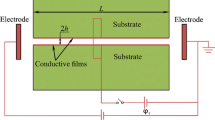
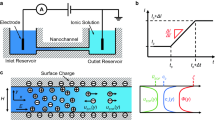
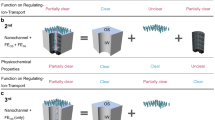
The electrokinetic conductivity of micro-/nanofluidic systems, which strongly depends on the local solution properties (e.g., pH and ionic strength), has wide applications in nanosystems to control the system performance and ion rectification. Accurate and active manipulation of this parameter is proven to be very challenging since, in nanoscale, the ion transport is particularly dominated by the acquired surface charge on the solid–liquid interfaces. In this study, we propose an approach to manipulate the nanochannel electrokinetic conductivity by changing the pH value of the solution at the inlet in order to impose asymmetrical conditions inside nanochannel. The variable surface charge of walls is determined by considering the chemical adsorption on the solid–liquid interface and the electrical double layer interaction. The presented numerical model, which couples Poisson–Nernst–Planck and Navier–Stokes equations, can fully consider the electro-chemo-mechanical transport phenomena and predict the electrokinetic conductivity of nanofluidic channels with good accuracy. Modeling results show that the electrokinetic conductivity of the nanofluidic systems can be regulated by varying the solution pH at the inlet. It is revealed that the stronger electric double layers interaction can enhance the sensitivity of the nanochannel electrokinetic conductance to the inlet pH. This unique behavior of the nanochannel electrokinetic conductivity could broaden potential applications in biomedical, energy, and environmental systems using nanofluidic devices.










Similar content being viewed by others
Abbreviations
- ETL:
-
Electrical triple layer
- METL:
-
Modified electrical triple layer, EDL, electrical double layer
- SCF:
-
Streaming conductance factor
- ECF:
-
Electrical conductance factor
References
Alizadeh A, Zhang L, Wang M (2014) Mixing enhancement of low-Reynolds electro-osmotic flows in microchannels with temperature-patterned walls. J Colloid Interface Sci 431:50–63
Baldessari F, Santiago JG (2009) Electrokinetics in nanochannels. Part I. Electric double layer overlap and channel-to-well equilibrium (vol 325, pg 526, 2008). J Colloid Interface Sci 331:549
Behrens SH, Grier DG (2001) The charge of glass and silica surfaces. J Chem Phys 115:6716–6721
Charmas R, Piasecki W, Rudzinski W (1995) 4-Layer complexation model for ion adsorption at electrolyte/oxide interface—theoretical foundations. Langmuir 11:3199–3210
Chen Y, Wang XH, Erramilli S, Mohanty P, Kalinowski A (2006) Silicon-based nanoelectronic field-effect pH sensor with local gate control. Appl Phys Lett 89:2392828
Cheng LJ, Guo LJ (2007) Rectified ion transport through concentration gradient in homogeneous silica nanochannels. Nano Lett 7:3165–3171
Daiguji H, Yang PD, Szeri AJ, Majumdar A (2004a) Electrochemomechanical energy conversion in nanofluidic channels. Nano Lett 4:2315–2321
Daiguji H, Yang PD, Majumdar A (2004b) Ion transport in nanofluidic channels. Nano Lett 4:137–142
Guan WH, Fan R, Reed MA (2011) Field-effect reconfigurable nanofluidic ionic diodes. Nat Commun 2:8
Hou X, Yang F, Li L, Song YL, Jiang L, Zhu DB (2010) A biomimetic asymmetric responsive single nanochannel. J Am Chem Soc 132:11736–11742
Howorka S, Siwy Z (2009) Nanopore analytics: sensing of single molecules. Chem Soc Rev 38:2360–2384
Jiang ZJ, Stein D (2010) Electrofluidic gating of a chemically reactive surface. Langmuir 26:8161–8173
Jiang ZJ, Stein D (2011) Charge regulation in nanopore ionic field-effect transistors. Phys Rev E 83:031203
Karnik R, Castelino K, Fan R, Yang P, Majumdar A (2005a) Effects of biological reactions and modifications on conductance of nanofluidic channels. Nano Lett 5:1638–1642
Karnik R, Fan R, Yue M, Li DY, Yang PD, Majumdar A (2005b) Electrostatic control of ions and molecules in nanofluidic transistors. Nano Lett 5:943–948
Karnik R, Duan C, Castelino K, Daiguji H, Majumdar A (2007) Rectification of ionic current in a nanofluidic diode. Nano Lett 7:547–551
Kitamura A, Fujiwara K, Yamamoto T, Nishikawa S, Moriyama H (1999) Analysis of adsorption behavior of cations onto quartz surface by electrical double-layer model. J Nucl Sci Technol 36:1167–1175
Lanju M, Li-Hsien Y, Shizhi Q (2015) Buffer effect on the ionic conductance in a pH-regulated nanochannel. Electrochem Commun 51:129–132
Li W, Bell NAW, Hernandez-Ainsa S, Thacker VV, Thackray AM, Bujdoso R, Keyser UF (2013) Single protein molecule detection by glass nanopores. ACS Nano 7:4129–4134
Lichtner PC (1995) Principles and practice of reactive transport modeling. In: Scientific basis for nuclear waste management XVII. Symposium, vol 111, pp 117–130
Li-Hsien Y, Yu M, Song X, Shizhi Q (2015) Gate manipulation of ionic conductance in a nanochannel with overlapped electric double layers. Sens Actuators B Chem 215:266–271
Ma Y, Xue S, Hsu SC, Yeh LH, Qian SZ, Tan HP (2014) Programmable ionic conductance in a pH-regulated gated nanochannel. Phys Chem Chem Phys 16:20138–20146
Ma Y, Yeh LH, Lin CY, Mei LJ, Qian SZ (2015) pH-regulated ionic conductance in a nanochannel with overlapped electric double layers. Anal Chem 87:4508–4514
Mao P, Han JY (2005) Fabrication and characterization of 20 nm planar nanofluidic channels by glass–glass and glass–silicon bonding. Lab Chip 5:837–844
Morgan H, Green NG (2003) AC electrokinetics: colloids and nanoparticles, 1st edn. Research Studies Press, UK
Pennathur S, Santiago JG (2005) Electrokinetic transport in nanochannels. 1. Theory. Anal Chem 77:6772–6781
Qiao R, Aluru NR (2004) Charge inversion and flow reversal in a nanochannel electro-osmotic flow. Phys Rev Lett 92:198301
Samson E, Marchand J, Snyder KA (2003) Calculation of ionic diffusion coefficients on the basis of migration test results. Mater Struct 36:156–165
Schoch RB, Renaud P (2005) Ion transport through nanoslits dominated by the effective surface charge. Appl Phys Lett 86:253111
Siwy Z, Gu Y, Spohr HA, Baur D, Wolf-Reber A, Spohr R, Apel P, Korchev YE (2002) Rectification and voltage gating of ion currents in a nanofabricated pore. Europhys Lett 60:349–355
Stein D, Kruithof M, Dekker C (2004) Surface-charge-governed ion transport in nanofluidic channels. Phys Rev Lett 93:035901
Stumm W, Morgan JJ (1996) Aquatic chemistry: chemical equilibria and rates in natural waters, 3rd edn. Wiley, London
Taghipoor M, Bertsch A, Renaud P (2015a) An improved model for predicting electrical conductance in nanochannels. Phys Chem Chem Phys 17:4160–4167
Taghipoor M, Bertsch A, Renaud P (2015b) Temperature sensitivity of nanochannel electrical conductance. ACS Nano 9:4563–4571
Thompson AP (2003) Nonequilibrium molecular dynamics simulation of electro-osmotic flow in a charged nanopore. J Chem Phys 119:7503–7511
van der Heyden FHJ, Stein D, Dekker C (2005) Streaming currents in a single nanofluidic channel. Phys Rev Lett 95:116104
van der Heyden FHJ, Bonthuis DJ, Stein D, Meyer C, Dekker C (2006) Electrokinetic energy conversion efficiency in nanofluidic channels. Nano Lett 6:2232–2237
van der Heyden FHJ, Bonthuis DJ, Stein D, Meyer C, Dekker C (2007) Power generation by pressure-driven transport of ions in nanofluidic channels. Nano Lett 7:1022–1025
Wang M, Chen S (2007) Electroosmosis in homogeneously charged micro- and nanoscale random porous media. J Colloid Interface Sci 314:264–273
Wang M, Kang Q (2010a) Electrochemomechanical energy conversion efficiency in silica nanochannels. Microfluid Nanofluid 9:181–190
Wang M, Kang Q (2010b) Modeling electrokinetic flows in microchannels using coupled lattice Boltzmann methods. J Comput Phys 229:728–744
Wang M, Revil A (2010) Electrochemical charge of silica surfaces at high ionic strength in narrow channels. J Colloid Interface Sci 343:381–386
Wang JK, Wang M, Li ZX (2006) Lattice Poisson–Boltzmann simulations of electro-osmotic flows in microchannels. J Colloid Interface Sci 296:729–736
Wang M, Liu J, Chen S (2007) Electric potential distribution in nanoscale electroosmosis: from molecules to continuum. Mol Simul 33:1273–1277
Wang L, Guo W, Xie YB, Wang XW, Xue JM, Wang YG (2009) Nanofluidic diode generated by pH gradient inside track-etched conical nanopore. Radiat Meas 44:1119–1122
Wang M, Kang Q, Ben-Naim E (2010) Modeling of electrokinetic transport in silica nanofluidic channels. Anal Chim Acta 664:158–164
Yan Y, Sheng Q, Wang C, Xue J, Chang H-C (2013) Energy conversion efficiency of nanofluidic batteries: hydrodynamic slip and access resistance. J Phys Chem C 117:8050–8061
Yeh L-H, Zhang M, Joo SW, Qian S, Hsu J-P (2012) Controlling pH-regulated bionanoparticles translocation through nanopores with polyelectrolyte brushes. Anal Chem 84:9615–9622
Acknowledgements
This work is financially supported by the NSF grant of China (Nos. 51676107, 51176089), National Science and Technology Major Project on Oil and Gas (No. 2017ZX05013001) and Tsinghua University Initiative Scientific Research Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the topical collection “2016 International Conference of Microfluidics, Nanofluidics and Lab-on-a-Chip, Dalian, China” guest edited by Chun Yang, Carolyn Ren and Xiangchun Xuan.
Rights and permissions
About this article
Cite this article
Alizadeh, A., Warkiani, M.E. & Wang, M. Manipulating electrokinetic conductance of nanofluidic channel by varying inlet pH of solution. Microfluid Nanofluid 21, 52 (2017). https://doi.org/10.1007/s10404-017-1892-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-017-1892-9