Abstract
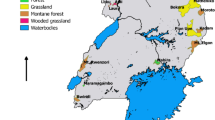
Rodent species were assessed as potential hosts of Trypanosoma cruzi, the etiologic agent of Chagas disease, from five sites throughout Texas in sylvan and disturbed habitats. A total of 592 rodents were captured, resulting in a wide taxonomic representation of 11 genera and 15 species. Heart samples of 543 individuals were successfully analyzed by SybrGreen-based quantitative PCR (qPCR) targeting a 166 bp fragment of satellite DNA of T. cruzi. Eight rodents representing six species from six genera and two families were infected with T. cruzi. This is the first report of T. cruzi in the pygmy mouse (Baiomys taylori) and the white-footed mouse (Peromyscus leucopus) for the USA. All infected rodents were from the southernmost site (Las Palomas Wildlife Management Area). No differences in pathogen prevalence existed between disturbed habitats (5 of 131 tested; 3.8%) and sylvan habitats (3 of 40 tested; 7.5%). Most positives (n = 6, 16% prevalence) were detected in late winter with single positives in both spring (3% prevalence) and fall (1% prevalence). Additionally, 30 Triatoma insects were collected opportunistically from sites in central Texas. Fifty percent of these insects, i.e., 13 T. gerstaeckeri (68%), and two T. lecticularia (100%) were positive for T. cruzi. Comparative sequence analyses of 18S rRNA of samples provided identical results with respect to detection of the presence or absence of T. cruzi and assigned T. cruzi from rodents collected in late winter to lineage TcI. T. cruzi from Triatoma sp. and rodents from subsequent collections in spring and fall were different, however, and could not be assigned to other lineages with certainty.



Similar content being viewed by others
References
Beard CB, Pye G, Steurer FJ, Rodriguez R, Campman R, Peterson AT, Ramsey J, Wirtz RA, Robinson LE (2003) Chagas disease in a domestic transmission cycle in southern Texas, USA. Emerging Infectious Diseases 9:103–105
Berger WH, Parker FL (1970) Diversity of planktonic foraminifera in deep-sea sediments. Science 168:1345–1347
Bern C, Kjos S, Yabsley MJ, Montgomery SP (2011) Trypanosoma cruzi and Chagas’ disease in the United States. Clinical Microbiology Reviews 24:655–681. doi:10.1128/Cmr.00005-11
Bern C, Montgomery SP (2009) An estimate of the burden of Chagas disease in the United States. Clinical Infectious Diseases 49:E52–E54. doi:10.1086/605091
Bosseno MF, Barnabe C, Gastelum EM, Kasten FL, Ramsey J, Espinoza B, Breniere SF (2002) Predominance of Trypanosoma cruzi lineage I in Mexico. Journal of Clinical Microbiology 40:627–632. doi:10.1128/Jcm.40.2.627-632.2002
Brown EL, Roellig DM, Gompper ME, Monello RJ, Wenning KM, Gabriel MW, Yabsley MJ (2010) Seroprevalence of Trypanosoma cruzi among eleven potential reservoir species from six states across the southern United States. Vector-Borne and Zoonotic Diseases 10:757–763. doi:10.1089/vbz.2009.0009
Burkholder JE, Allison TC, Kelly VP (1980) Trypanosoma cruzi (Chagas) (Protozoa: Kinetoplastida) in invertebrate, reservoir, and human host of the lower Rio Grande Valley of Texas. Journal of Parasitology 66:305–311
Caldas S, Caldas IS, Diniz LD, de Lima WG, Oliveira RD, Cecilio AB, Ribeiro I, Talvani A, Bahia MT (2012) Real-time PCR strategy for parasite quantification in blood and tissue samples of experimental Trypanosoma cruzi infection. Acta Tropica 123:170–177. doi:10.1016/j.actatropica.2012.05.002
Canals M, Solis R, Tapia C, Ehrenfeld M, Cattan PE (1999) Comparison of some behavioral and physiological feeding parameters of Triatoma infestans Klug, 1834 and Mepraia spinolai Porter, 1934, vectors of Chagas disease in Chile. Memorias Do Instituto Oswaldo Cruz 94:687–692
Cantey PT, Stramer SL, Townsend RL, Kamel H, Ofafa K, Todd CW, Currier M, Hand S, Varnado W, Dotson E, Hall C, Jett PL, Montgomery SP (2012) The United States Trypanosoma cruzi infection study: evidence for vector-borne transmission of the parasite that causes Chagas disease among United States blood donors. Transfusion 52:1922–1930. doi:10.1111/j.1537-2995.2012.03581.x
Centers for Disease Control and Prevention (CDC) (2016) Parasites—American Trypanosomiasis (also known as Chagas Disease). http://www.cdc.gov/parasites/chagas
Charles RA, Kjos S, Ellis AE, Barnes JC, Yabsley MJ (2013) Southern plains woodrats (Neotoma micropus) from southern Texas are important reservoirs of two genotypes of Trypanosoma cruzi and host of a putative novel Trypanosoma species. Vector-Borne and Zoonotic Diseases 13:22–30. doi:10.1089/vbz.2011.0817
Curtis-Robles R, Lewis BC, Hamer SA (2016) High Trypanosoma cruzi infection prevalence associated with minimal cardiac pathology among wild carnivores in central Texas. International Journal for Parasitology: Parasites and Wildlife 5:117–123
Curtis-Robles R, Wozniak EJ, Auckland LD, Hamer GL, Hamer SA (2015) Combining public health education and disease ecology research: using citizen science to assess Chagas disease entomological risk in Texas. Plos Neglected Tropical Diseases 9. doi:10.1371/journal.pntd.0004235
Davis WB, Schmidly DJ (1994) The Mammals of Texas. Austin, TX: Texas Parks and Wildlife Press.
Dorn PL, Perniciaro L, Yabsley MJ, Roellig DM, Balsamo G, Diaz J, Wesson D (2007) Autochthonous transmission of Trypanosoma cruzi, Louisiana. Emerging Infectious Diseases 13:605–607
Duffy T, Cura CI, Ramirez JC, Abate T, Cayo NM, Parrado R, Bello ZD, Velazquez E, Munoz-Calderon A, Juiz NA, Basile J, Garcia L, Riarte A, Nasser JR, Ocampo SB, Yadon ZE, Torrico F, de Noya BA, Ribeiro I, Schijman AG (2013) Analytical performance of a multiplex real-time PCR assay using TaqMan probes for quantification of Trypanosoma cruzi satellite DNA in blood samples. Plos Neglected Tropical Diseases 7. doi:10.1371/journal.pntd.0002000
Elias MCQB, Vargas N, Tomazi L, Pedroso A, Zingales B, Schenkman S, Briones MRS (2005) Comparative analysis of genomic sequences suggests that Trypanosoma cruzi CL Brener contains two sets of non-intercalated repeats of satellite DNA that correspond to T. cruzi I and T. cruzi II types. Molecular and Biochemical Parasitology 140:221–227. doi:10.1016/j.molbiopara.2004.12.016
Felsenstein J (1981) Evolutionary trees from DNA sequences: a maximum likelihood approach. Journal of Molecular Evolution 17:368–376
Felsenstein J (1985) Confidence limits of phylogenies: an approach using the bootstrap. Evolution 39:783–791
Gal AB, Carnwath JW, Dinnyes A, Herrmann D, Niemann H, Wrenzycki C (2006) Comparison of real-time polymerase chain reaction and end-point polymerase chain reaction for the analysis of gene expression in preimplantation embryos. Reproduction Fertility and Development 18:365–371. doi:10.1071/Rd05012
Garcia MN, Aguilar D, Gorchakov R, Rossmann SN, Montgomery SP, Rivera H, Woc-Colburn L, Hotez PJ, Murray KO (2015) Case report: evidence of autochthonous chagas disease in southeastern Texas. American Journal of Tropical Medicine and Hygiene 92:325–330. doi:10.4269/ajtmh.14-0238
Garza M, Arroyo TPF, Casillas EA, Sanchez-Cordero V, Rivaldi CL, Sarkar S (2014) Projected future distributions of vectors of Trypanosoma cruzi in North America under climate change scenarios. Plos Neglected Tropical Diseases 8. doi:10.1371/journal.pntd.0002818
Gascon J, Bern C, Pinazo MJ (2010) Chagas disease in Spain, the United States and other non-endemic countries. Acta Tropica 115:22–27. doi:10.1016/j.actatropica.2009.07.019
Gottdenker NL, Chaves LF, Calzada JE, Saldana A, Carroll CR (2012) Host life history strategy, species diversity, and habitat influence Trypanosoma cruzi vector infection in changing landscapes. Plos Neglected Tropical Diseases 6. doi:10.1371/journal.pntd.0001884
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology 59:307–321
Hanford EJ, Zhan FB, Lu YM, Giordano A (2007) Chagas disease in Texas: recognizing the significance and implications of evidence in the literature. Social Science and Medicine 65:60–79. doi:10.1016/j.socscimed.2007.02.041
Herrera CP, Licon MH, Nation CS, Jameson SB, Wesson DM (2015) Genotype diversity of Trypanosoma cruzi in small rodents and Triatoma sanguisuga from a rural area in New Orleans, Louisiana. Parasites and Vectors 8. doi:10.1186/s13071-015-0730-8
Herwaldt BL, Grijalva MJ, Newsome AL, McGhee CR, Powell MR, Nemec DG, Steurer FJ, Eberhard ML (2000) Use of polymerase chain reaction to diagnose the fifth reported US case of autochthonous transmission of Trypanosoma cruzi, in Tennessee, 1998. Journal of Infectious Diseases 181:395–399. doi:10.1086/315212
Huang ZYX, Langevelde FV, Estrada-Pena A, Suzan G, de Boer WF (2016) The diversity-disease relationship: evidence for and criticisms of the dilution effect. Parasitology. doi:10.1017/S0031182016000536
Hurlbert SH (1971) The nonconcept of species diversity: a critique and alternative parameters. Ecology 52:577–585
Johnson PTJ, De Roode JC, Fenton A (2015) Why infectious disease research needs community ecology. Science 349. doi:10.1126/science.1259504
Justi SA, Russo CAM, Mallet JRD, Obara MT, Galvao C (2014) Molecular phylogeny of Triatomini (Hemiptera: Reduviidae: Triatominae). Parasites & Vectors 7. doi:10.1186/1756-3305-7-149
Kirchhoff LV (1993) American Trypanosomiasis (Chagas’ disease)—a tropical disease now in the United States. The New England Journal of Medicine 329:639–644
Kjos SA, Marcet PL, Yabsley MJ, Kitron U, Snowden KF, Logan KS, Barnes JC, Dotson EM (2013) Identification of bloodmeal sources and Trypanosoma cruzi infection in triatomine bugs (Hemiptera: Reduviidae) from residential settings in Texas, the United States. Journal of Medical Entomology 50:1126–1139. doi:10.1603/Me12242
Kjos SA, Snowden KF, Craig TM, Lewis B, Ronald N, Olson JK (2008) Distribution and characterization of canine Chagas disease in Texas. Veterinary Parasitology 152:249–256. doi:10.1016/j.vetpar.2007.12.021
Kjos SA, Snowden KF, Olson JK (2009) Biogeography and Trypanosoma cruzi infection prevalence of Chagas disease vectors in Texas, USA. Vector-Borne and Zoonotic Diseases 9:41–49. doi:10.1089/vbz.2008.0026
Lambert RC, Kolivras KN, Resler LM, Brewster CC, Paulson SL (2008) The potential for emergence of Chagas disease in the United States. Geospatial Health 2:227–239
Leite GR, dos Santos CB, Falqueto A (2011) Influence of the landscape on dispersal of sylvatic triatomines to anthropic habitats in the Atlantic Forest. Journal of Biogeography 38:651–663. doi:10.1111/j.1365-2699.2010.02442.x
LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F (2003) The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proceedings of the National Academy of Sciences of the United States of America 100:567–571. doi:10.1073/pnas.0233733100
Maikis TJ (2014) Detection of Borrelia burgdorferi Sensu Lato Infection in Rodents from Disturbed and Sylvan Assemblages Across Texas. San Marcos, TX: Texas State University.
Mills JN, Gage KL, Khan AS (2010) Potential influence of climate change on vector-borne and zoonotic diseases: a review and proposed research plan. Environmental Health Perspectives 118:1507–1514. doi:10.1289/ehp.0901389
Monroy C, Rodas A, Mejia M, Rosales R, Tabaru Y (2003) Epidemiology of Chagas disease in Guatemala: infection rate of Triatoma dimidiata, Triatoma nitida and Rhodnius prolixus (Hemiptera, Reduviidae) with Trypanosoma cruzi and Trypanosoma rangeli (Kinetoplastida, Trypanosomatidae). Memorias Do Instituto Oswaldo Cruz 98:305–310. doi:10.1590/S0074-02762003000300003
Moreira OC, Ramirez JD, Velazquez E, Melo MFAD, Lima-Ferreira C, Guhl F, Sosa-Estani S, Marin-Neto JA, Morillo CA, Britto C (2013) Towards the establishment of a consensus real-time qPCR to monitor Trypanosoma cruzi parasitemia in patients with chronic Chagas disease cardiomyopathy: a substudy from the BENEFIT trial. Acta Tropica 125:23–31. doi:10.1016/j.actatropica.2012.08.020
Mubiru JN, Yang A, Dick EJ, Owston M, Sharp RM, VandeBerg JF, Shade RE, VandeBerg JL (2014) Correlation between presence of Trypanosoma cruzi DNA in heart tissue of baboons and cynomolgus monkeys, and lymphocytic myocarditis. American Journal of Tropical Medicine and Hygiene 90:627–633. doi:10.4269/ajtmh.13-0448
Murphy WJ, O’Brien SJ (2007) Designing and optimizing comparative anchor primers for comparative gene mapping and phylogenetic inference. Nature Protocols 2:3022–3030. doi:10.1038/nprot.2007.429
Noyes HA, Stevens JR, Teixeira M, Phelan J Holz P (1999) A nested PCR for the ssrRNA gene detects Trypanosoma binneyi in the platypus and Trypanosoma sp. in wombats and kangaroos in Australia. International Journal for Parasitology 29:331–339. doi:10.1016/S0020-7519(98)00167-2
Nylander JAA (2004) MrModeltest 2.0. Uppsala: Evolutionary Biology Centre, Uppsala University.
Ocaña-Mayorga S, Aguirre-Villacis F, Pinto CM, Vallejo GA, Grijalva MJ (2015) Prevalence, genetic characterization, and 18S small subunit ribosomal RNA diversity of Trypanosoma rangeli in triatomine and mammal hosts in endemic areas for Chagas disease in Ecuador. Vector-Borne and Zoonotic Diseases 15:732–742
Patel JM, Rosypal AC, Zimmerman KL, Monroe WE, Sriranganathan N, Zajac AM, Yabsley ML, Lindsay DS (2012) Isolation, mouse pathogenicity, and genotyping of Trypanosoma cruzi from an English Cocker Spaniel from Virginia, USA. Veterinary Parasitology 187:394–398. doi:10.1016/j.vetpar.2012.01.031
Pearson WR Lipman DJ (1988) Improved tools for biological sequence comparison. Proceedings of the National Academy of Sciences of the United States of America 85:2444–2448
Pfeiler E, Bitler BG, Ramsey JM, Palacios-Cardiel C, Markow TA (2006) Genetic variation, population structure, and phylogenetic relationships of Triatoma rubida and T. recurva (Hemiptera:Reduviidae:Triatominae) from the Sonoran Desert, insect vectors of the Chagas’ disease parasite Trypanosoma cruzi. Molecular Phylogenetics and Evolution 41:209–221. doi:10.1016/j.ympev.2006.07.001
Pielou EC (1975) Mathematical Ecology. New York: Wiley.
Pinto CM, Kalko EKV, Cottontail L, Wellinghausen N, Cottontail VM (2012) TcBat a bat-exclusive lineage of Trypanosoma cruzi in the Panama Canal Zone, with comments on its classification and the use of the 18S rRNA gene for lineage identification. Infection Genetics and Evolution 12:1328–1332. doi:10.1016/j.meegid.2012.04.013
Pinto CM, Ocana-Mayorga S, Tapia EE, Lobos SE, Zurita AP, Aguirre-Villacis F, MacDonald A, Villacis AG, Lima L, Teixeira MMG, Grijalva MJ, Perkins SL (2015) Bats, trypanosomes, and triatomines in Ecuador: new insights into the diversity, transmission, and origins of Trypanosoma cruzi and Chagas Disease. Plos One 10. doi:10.1371/journal.pone.0139999
Piron M, Fisa R, Casamitjana N, Lopez-Chejade P, Puig L, Verges M, Gascon J, Prat JGI, Portus M, Sauleda S (2007) Development of a real-time PCR assay for Trypanosoma cruzi detection in blood samples. Acta Tropica 103:195–200. doi:10.1016/j.actatropica.2007.05.019
Posada D, Crandell KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14:817–818
Rassi A, Jr., Rassi A, Little WC (2000) Chagas’ heart disease. Clinical Cardiology 23:883–889
Ronquist F, Huelsenbeck JP (2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Rosypal AC, Hill R, Lewis S, Barr SC, Valadast S, Gennari SM, Lindsay DS (2011) Evaluation of a rapid immunochromatographic dipstick test for detection of antibodies to Trypanosoma cruzi in dogs experimentally infected with isolates obtained from Opossums (Didelphis virginiana), Armadillos (Dasypus novemcinctus), and Dogs (Canis familiaris) from the United States. Journal of Parasitology 97:140–143. doi:10.1645/Ge-2559.1
Sainz AC, Mauro LV, Moriyama EN, Garcia BA (2004) Phylogeny of triatomine vectors of Trypanosoma cruzi suggested by mitochondrial DNA sequences. Genetica 121:229–240. doi:10.1023/B:Gene.0000039842.82574.02
Sarkar S, Strutz SE, Frank DM, Rivaldi CL, Sissel B, Sanchez-Cordero V (2010) Chagas disease risk in Texas. Plos Neglected Tropical Diseases 4. doi:10.1371/journal.pntd.0000836
Schiffler RJ, Mansur GP, Navin TR, Limpakarnjanarat K (1984) Indigenous Chagas-disease (American Trypanosomiasis) in California. Jama-Journal of the American Medical Association 251:2983–2984. doi:10.1001/jama.251.22.2983
Schofield CJ, Galvao C (2009) Classification, evolution, and species groups within the Triatominae. Acta Tropica 110:88–100. doi:10.1016/j.actatropica.2009.01.010
Soares RPP, Evangelista LD, Laranja LS, Diotaiuti L (2000) Population dynamics and feeding behavior of Triatoma brasiliensis and Triatoma pseudomaculata, main vectors of Chagas disease in Northeastern Brazil. Memorias Do Instituto Oswaldo Cruz 95:151–155. doi:10.1590/S0074-02762000000200003
Suzan G, Garcia-Pena GE, Castro-Arellano I, Rico O, Rubio AV, Tolsa MJ, Roche B, Hosseini PR, Rizzoli A, Murray KA, Zambrana-Torrelio C, Vittecoq M, Bailly X, Aguirre AA, Daszak P, Prieur-Richard AH, Mills JN, Guegan JF (2015) Metacommunity and phylogenetic structure determine wildlife and zoonotic infectious disease patterns in time and space. Ecology and Evolution 5:865–873. doi:10.1002/ece3.1404
Swofford DL (2002) PAUP*. Phylogenetic Analysis Using Parsimony (* and other Methods), Version 4. Sunderland: Sinauer Associates.
Tenney TD, Curtis-Robles R, Snowden KF, Hamer SA (2014) Shelter dogs as sentinels for Trypanosoma cruzi Transmission across Texas, USA. Emerging Infectious Diseases 20:1323–1326. doi:10.3201/eid2008.131843
Van der Pol B (2007) Trichomonas vaginalis infection: the most prevalent nonviral sexually transmitted infection receives the least public health attention. Clinical Infectious Diseases 44:23–25
Virreira M, Torrico F, Truyens C, Alonso-Vega C, Solano M, Carlier Y, Svoboda M (2003) Comparison of polymerase chain reaction methods for reliable and easy detection of congenital Trypanosoma cruzi infection. American Journal of Tropical Medicine and Hygiene 68:574–582
Waleckx E, Suarez J, Richards B, Dorn PL (2014) Triatoma sanguisuga blood meals and potential for Chagas disease, Louisiana, USA. Emerging Infectious Diseases 20:2141–2143. doi:10.3201/eid2012.131576
Wilson DE, Reeder DM (2005) Mammal species of the world: a taxonomic and geographic reference, 3rd ed. Baltimore, MD: John Hopkins University Press.
World Health Organization (WHO) (2016) Chagas disease (American trypanosomiasis). http://www.who.int/mediacentre/factsheets/fs340/en/
Wozniak EJ, Lawrence G, Gorchakov R, Alamgir H, Dotson E, Sissel B, Sarkar S, Murray KO (2015) The biology of the triatomine bugs native to South Central Texas and assessment of the risk they pose for autochthonous Chagas disease exposure. Journal of Parasitology 101:520–528. doi:10.1645/15-748
Zingales B, Andrade SG, Briones MRS, Campbell DA, Chiari E, Fernandes O, Guhl F, Lages-Silva E, Macedo AM, Machado CR, Miles MA, Romanha AJ, Sturm NR, Tibayrenc M, Schijman AG (2009) A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Memorias Do Instituto Oswaldo Cruz 104:1051–1054
Acknowledgements
Collection and handling of rodents was covered by Texas State University IACUC permit 1206_0113_02. We are grateful to Dr. J.M. Ramsey Willoquet, Regional Center for Public Health Research, National Institute for Public Health Research, Tapachula, Chiapas, Mexico, for providing DNA of T. cruzi ITRI/MX/99/Cari-006, and the United States Department of Agriculture (USDA) for financial support (NIFA 201 3-70001-20524).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aleman, A., Guerra, T., Maikis, T.J. et al. The Prevalence of Trypanosoma cruzi, the Causal Agent of Chagas Disease, in Texas Rodent Populations. EcoHealth 14, 130–143 (2017). https://doi.org/10.1007/s10393-017-1205-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-017-1205-5