Abstract
Aim
The study sought to determine the risk factors involved in Leptospira infection in rural settings and the relative severity of the disease in comparison with the urban environment in Tiruchirappalli district, Tamil Nadu, India.
Subject and methods
During an upsurge of leptospirosis, 621 suspected blood samples were obtained from patients and screened by MAT and IgM ELISA. To set up an age-matched case control study, 1,230 seronegative control samples were also included in the study. The case-control analysis was carried out using unconditional logistic regression and all P-values were two-sided.
Results
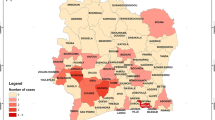
From 621 suspected cases, 214 (74 %) from rural and 196 (59 %) from urban settings had a laboratory-confirmed diagnosis of leptospirosis. The highest antibody titres were identified against Leptospira interrogans serovar Canicola both in rural (35 %) and urban (38 %) settings. Increased infection risk was observed for cases in the age group of 20–40 years and 10–40 years in rural and urban settings respectively. In rural settings, the risk of leptospiral infection was found to be significantly (P < 0.0001) associated with people having cattle at their house and those involved in wet cultivation. Among urban cases, building workers and people living in house dwelling near water bodies were significantly (P < 0.0001) associated with leptospiral infection.
Conclusion
The findings of the present study identified ideal niches for the transmission of leptospires in the rural and urban settings. Identification and understanding the potential risk factors in different environments would help to identify the source of contamination. This also identifies the need for more public health assistance.


Similar content being viewed by others
References
Barcellos C, Sabroza PC (2001) The place behind the case: leptospirosis risk and associated environmental conditions in a flood-related outbreak in Rio de Janeiro. Cad Saude Publica 17:59–67
Dean AG, Dean JA, Coulombier D, Brendel KA, Smith DC, Burton AH (2009) Epi Info, Version 6: a word processing, database, and statistics program for epidemiology on microcomputers. Centers for Disease Control and Prevention, Atlanta, GA
Faine S (1982) Guidelines for control of leptospirosis. World Health Organization, Geneva
Faine S (1994) Leptospira and leptospirosis. CRC, London
Kmety E, Dikken H (1993) Classification of the species Leptospira interrogans and history of its serovars. University Press, Groningen, The Netherlands
Ko AI, Reis MG, Dourado CMR, Johnson WD Jr, Riley LW (1999) Urban epidemic of severe leptospirosis in Brazil. Lancet 354:820–825
Koteeswaran (2006) Seroprevalence of leptospirosis in man and animals in Tamilnadu. Indian J Med Microbiol 24:329–331
Levett PN (2001) Leptospirosis. Clin Microbiol Rev 14:296–326
Maciel EAP, Carvalho ALF, Nascimeto SF, Matos RB, Gouveia EL (2008) Household transmission of Leptospira infection in urban slum communities. PLoS Negl Trop Dis 2:e154
Murhekar MV, Sugunan AP, Vijayachari P, Sharma S, Seghal SC (1998) Risk factors in the transmission of leptospiral infection. Indian J Med Res 10:218–223
Natarajaseenivasan K, Ratnam S (1997) Seroprevalence of leptospiral infection in an agriculture based village in Tamilnadu. Cheiron 68:139–140
Natarajaseenivasan K, Prabhu N, Selvanayaki K, Raja SSS, Ratnam S (2004) Human leptospirosis in Erode, South India: serology, isolation and characterization of the isolates by randomly amplified polymorphic DNA (RAPD) fingerprinting. Jpn J Infect Dis 57:193–197
Natarajaseenivasan K, Vijayachari P, Sharma S, Roy S, Sugunan AP, Biswas D, Seghal SC (2005) Phylogenetic relatedness among leptospiral strains belonging to same serovar recovered from patients with different clinical syndromes. Infect Genet Evol 5:185–191
Natarajaseenivasan K, Vedhagiri K, Sivabalan V, Prabagaran SG, Sukumar S, Artiushin SC, Timoney JF (2011) Seroprevalence of Leptospira borgpetersenii serovar Javanica infection among dairy cattle, rats and humans in the Cauvery River valley of southern India. Southeast Asian J Trop Med Public Health 42:679–686
Ratnam S, Subramanian S, Madanagopalan N, Sundararaj T, Jayanthi V (1983a) Isolation of leptospirosis and demonstration of antibodies in human leptospirosis in Madras, India. Trans R Soc Trop Med Hyg 77:455–458
Ratnam S, Sundararaj T, Subramanian S (1983b) Serological evidence of leptospirosis in a human population following an outbreak of the disease in cattle. Trans R Soc Trop Med Hyg 77:94–98
Reis RB, Ribeiro GS, Felzemburgh RDB, Santana FS, Mohr S (2008) Impact of environment and social gradient on Leptospira infection in urban slums. PLoS Negl Trop Dis 2:e228
Sahai H, Khurshid A (1996) Statistics in epidemiology: methods, techniques and applications. CRC, Boca Raton, FL
Sehgal SC, Murhekar MV, Sugunan AP (1995) Outbreak of leptospirosis with pulmonary involvement in North Andaman. Indian J Med Res 102:9–12
Sharma S, Vijayachari P, Sugunan AP, Natarajaseenivasan K, Sehgal SC (2006) Seroprevalence of leptospirosis among high-risk population of Andaman Islands, India. Am J Trop Med Hyg 74:278–283
Singh SS, Vijayachari P, Sinha A, Sugunana AP, Rasheed MA, Sehgal SC (1999) Clinico-epidemiological study of hospitalized cases of severe leptospirosis. Indian J Med Res 109:94–99
Sugunan AP, Vijayachari P, Sharma S, Roy S, Manickam P, Natarajaseenivasan K, Gupte MD, Sehgal SC (2009) Risk factors associated with leptospirosis during an outbreak in Middle Andaman, India. Indian J Med Res 130:67–73
Tangkanakul W, Tharmaphornpil P, Plikaytis BD, Bragg S, Poonsuksombat D, Choomkasien P (2000) Risk factors associated with leptospirosis in northeastern Thailand, 1998. Am J Trop Med Hyg 63:204–208
Terpstra WJ, Lighthart GS, Schoone CJ (1985) ELISA for the detection of specific IgM and IgG in human leptospirosis. J Gen Microbiol 131:377–385
Vijayachari P, Sugunan AP, Sehgal SC (2001) Role of microscopic agglutination test (MAT) as a diagnostic tool during acute stage of leptospirosis in low and high endemic areas. Indian J Med Res 11:99–106
Zaki SR, Sheih WJ (1995) Leptospirosis associated with outbreak of acute febrile illness with pulmonary haemorrhage, Nicaragua, 1995: the epidemic working group at Ministry of Health in Nicaragua. Lancet 347:535–536
Acknowledgements
We thank the Indian Council of Medical Research (ICMR), New Delhi for the financial assistance (Sanction order No: 5/3/3/3/2007-ECD-I) to carry out this study. We also like to thank the clinicians and staff of Annal Gandhi Memorial Government Hospital, Tiruchirappalli and Government Hospital, Lalgudi for their assistance in conducting the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prabhakaran, S.G., Shanmughapriya, S., Dhanapaul, S. et al. Risk factors associated with rural and urban epidemics of leptospirosis in Tiruchirappalli District of Tamilnadu, India. J Public Health 22, 323–333 (2014). https://doi.org/10.1007/s10389-014-0611-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10389-014-0611-1