Abstract
Purpose
To examine the long-term natural course of retinal degeneration in rd10 and rd12 mice using serial spectral-domain optical coherence tomography (SD-OCT), electroretinography/electroretinograms (ERGs), and histological analysis.
Methods
Photoreceptor layer thickness and the ability to visualize photoreceptor ellipsoid zones were analyzed using SD-OCT images, and these images were compared with hematoxylin and eosin-stained sections and electron microscopy images. The a- and b-wave amplitudes of the ERGs were analyzed.
Results
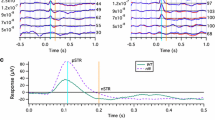
In rd10 mice, the photoreceptor layer thickness rapidly decreased, and the photoreceptor ellipsoid zone was visible on SD-OCT images in 89 and 43 % of eyes of 21 and 33-day-old mice, respectively. In rd12 mice, the photoreceptor layer gradually thinned, and the ellipsoid zone remained visible in 92 % of eyes at 19 months. Electron microscopy revealed that photoreceptor degeneration had occurred on the inner side of the outer nuclear layer in 21-day-old rd10 and 7-month-old rd12 mice, possibly due to autophagy mechanisms. Scotopic ERGs of rd10 mice showed a diminished response at 21 days; at 33 days, no response was detectable. In rd12 mice, scotopic ERGs were undetectable at 28 days (stimulus intensity 3.0 cds/m2). Photopic ERGs were nearly undetectable in 28-day-old rd10 mice, but a small b-wave was still recordable in 13-month-old rd12 mice.
Conclusions
Our results demonstrate that visual function deteriorated with photoreceptor degeneration within 1 month in rd10 mice. In rd12 mice, however, the process of visual function deterioration and photoreceptor degeneration was still in progress at 13 months of age.






Similar content being viewed by others
References
Shintani K, Shechtman DL, Gurwood AS. Review and update: current treatment trends for patients with retinitis pigmentosa. Optometry. 2009;80:384–401.
Ng TK, Fortino VR, Pelaez D, Cheung HS. Progress of mesenchymal stem cell therapy for neural and retinal diseases. World J Stem Cells. 2014;6:111–9.
Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–5.
Cai X, Conley SM, Naash MI. RPE65: role in the visual cycle, human retinal disease, and gene therapy. Ophthalmic Genet. 2009;30:57–62.
Sieving PA, Caruso RC, Tao W, Coleman HR, Thompson DJ, Fullmer KR, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci USA. 2006;103:3896–901.
Yang PB, Seiler MJ, Aramant RB, Yan F, Mahoney MJ, Kitzes LM, et al. Trophic factors GDNF and BDNF improve function of retinal sheet transplants. Exp Eye Res. 2010;91:727–38.
Yang Y, Mohand-Said S, Danan A, Simonutti M, Fontaine V, Clerin E, et al. Functional cone rescue by RdCVF protein in a dominant model of retinitis pigmentosa. Mol Ther. 2009;17:787–95.
Chizzolini M, Galan A, Milan E, Sebastiani A, Costagliola C, Parmeggiani F. Good epidemiologic practice in retinitis pigmentosa: from phenotyping to biobanking. Curr Genomics. 2011;12:260–6.
Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–809.
Oishi M, Oishi A, Gotoh N, Ogino K, Higasa K, Iida K, et al. Comprehensive molecular diagnosis of a large cohort of Japanese retinitis pigmentosa and usher syndrome patients by next-generation sequencing. Invest Ophthalmol Vis Sci. 2014;55:7369–75.
den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419.
Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–9.
Bennett J, Ashtari M, Wellman J, Marshall KA, Cyckowski LL, Chung DC, et al. AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med. 2012;4:120ra15.
Maguire AM, Simonelli F, Pierce EA, Pugh EN Jr, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358:2240–8.
Chang B, Hawes NL, Pardue MT, German AM, Hurd RE, Davisson MT, et al. Two mouse retinal degenerations caused by missense mutations in the beta-subunit of rod cGMP phosphodiesterase gene. Vis Res. 2007;47:624–33.
Rivas MA, Vecino E. Animal models and different therapies for treatment of retinitis pigmentosa. Histol Histopathol. 2009;24:1295–322.
Pang JJ, Chang B, Hawes NL, Hurd RE, Davisson MT, Li J, et al. Retinal degeneration 12 (rd12): a new, spontaneously arising mouse model for human Leber congenital amaurosis (LCA). Mol Vis. 2005;11:152–62.
Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–51.
Zheng Q, Ren Y, Tzekov R, Zhang Y, Chen B, Hou J, et al. Differential proteomics and functional research following gene therapy in a mouse model of Leber congenital amaurosis. PLoS ONE. 2012;7:e44855.
Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254:1178–81.
Jaffe GJ, Caprioli J. Optical coherence tomography to detect and manage retinal disease and glaucoma. Am J Ophthalmol. 2004;137:156–69.
Hangai M, Yamamoto M, Sakamoto A, Yoshimura N. Ultrahigh-resolution versus speckle noise-reduction in spectral-domain optical coherence tomography. Opt Express. 2009;17:4221–35.
Huber G, Beck SC, Grimm C, Sahaboglu-Tekgoz A, Paquet-Durand F, Wenzel A, et al. Spectral domain optical coherence tomography in mouse models of retinal degeneration. Invest Ophthalmol Vis Sci. 2009;50:5888–95.
Kim KH, Puoris’haag M, Maguluri GN, Umino Y, Cusato K, Barlow RB, et al. Monitoring mouse retinal degeneration with high-resolution spectral-domain optical coherence tomography. J Vis. 2008;8:17.1–17.11.
Pang JJ, Dai X, Boye SE, Barone I, Boye SL, Mao S, et al. Long-term retinal function and structure rescue using capsid mutant AAV8 vector in the rd10 mouse, a model of recessive retinitis pigmentosa. Mol Ther. 2011;19:234–42.
Pennesi ME, Michaels KV, Magee SS, Maricle A, Davin SP, Garg AK, et al. Long-term characterization of retinal degeneration in rd1 and rd10 mice using spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:4644–56.
Ruggeri M, Wehbe H, Jiao S, Gregori G, Jockovich ME, Hackam A, et al. In vivo three-dimensional high-resolution imaging of rodent retina with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2007;48:1808–14.
Wang R, Jiang C, Ma J, Young MJ. Monitoring morphological changes in the retina of rhodopsin-/- mice with spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:3967–72.
Nakano N, Ikeda HO, Hangai M, Muraoka Y, Toda Y, Kakizuka A, et al. Longitudinal and simultaneous imaging of retinal ganglion cells and inner retinal layers in a mouse model of glaucoma induced by N-methyl-d-aspartate. Invest Ophthalmol Vis Sci. 2011;52:8754–62.
Muraoka Y, Ikeda HO, Nakano N, Hangai M, Toda Y, Okamoto-Furuta K, et al. Real-time imaging of rabbit retina with retinal degeneration by using spectral-domain optical coherence tomography. PLoS ONE. 2012;7:e36135.
Marmor MF, Fulton AB, Holder GE, Miyake Y, Brigell M, Bach M. ISCEV standard for full-field clinical electroretinography (2008 update). Doc Ophthalmol. 2009;118:69–77.
Staurenghi G, Sadda S, Chakravarthy U, Spaide RF. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the IN*OCT consensus. Ophthalmology. 2014;121:1572–8.
Wong IY, Iu LP, Koizumi H, Lai WW. The inner segment/outer segment junction: what have we learnt so far? Curr Opin Ophthalmol. 2012;23:210–8.
Oishi A, Hata M, Shimozono M, Mandai M, Nishida A, Kurimoto Y. The significance of external limiting membrane status for visual acuity in age-related macular degeneration. Am J Ophthalmol. 2010;150(27–32):e1.
Breton ME, Schueller AW, Lamb TD, Pugh EN Jr. Analysis of ERG a-wave amplification and kinetics in terms of the G-protein cascade of phototransduction. Invest Ophthalmol Vis Sci. 1994;35:295–309.
Hood DC, Birch DG. Beta wave of the scotopic (rod) electroretinogram as a measure of the activity of human on-bipolar cells. J Opt Soc Am A Opt Image Sci Vis. 1996;13:623–33.
Korol S, Leuenberger PM, Englert U, Babel J. In vivo effects of glycine on retinal ultrastructure and averaged electroretinogram. Brain Res. 1975;97:235–51.
Jacobson SG, Aleman TS, Cideciyan AV, Sumaroka A, Schwartz SB, Windsor EA, et al. Identifying photoreceptors in blind eyes caused by RPE65 mutations: prerequisite for human gene therapy success. Proc Natl Acad Sci USA. 2005;102:6177–82.
Seeliger MW, Grimm C, Stahlberg F, Friedburg C, Jaissle G, Zrenner E, et al. New views on RPE65 deficiency: the rod system is the source of vision in a mouse model of Leber congenital amaurosis. Nat Genet. 2001;29:70–4.
Kuroda M, Hirami Y, Hata M, Mandai M, Takahashi M, Kurimoto Y. Intraretinal hyperreflective foci on spectral-domain optical coherence tomographic images of patients with retinitis pigmentosa. Clin Ophthalmol. 2014;8:435–40.
Bo Q, Ma S, Han Q, Wang FE, Li X, Zhang Y. Role of autophagy in photoreceptor cell survival and death. Crit Rev Eukaryot Gene Expr. 2015;25:23–32.
Sancho-Pelluz J, Arango-Gonzalez B, Kustermann S, Romero FJ, van Veen T, Zrenner E, et al. Photoreceptor cell death mechanisms in inherited retinal degeneration. Mol Neurobiol. 2008;38:253–69.
Newsome DA, Michels RG. Detection of lymphocytes in the vitreous gel of patients with retinitis pigmentosa. Am J Ophthalmol. 1988;105:596–602.
Yoshida N, Ikeda Y, Notomi S, Ishikawa K, Murakami Y, Hisatomi T, et al. Clinical evidence of sustained chronic inflammatory reaction in retinitis pigmentosa. Ophthalmology. 2013;120:100–5.
Yoshida N, Ikeda Y, Notomi S, Ishikawa K, Murakami Y, Hisatomi T, et al. Laboratory evidence of sustained chronic inflammatory reaction in retinitis pigmentosa. Ophthalmology. 2013;120:e5–12.
Sahel J, Bonnel S, Mrejen S, Paques M. Retinitis pigmentosa and other dystrophies. Dev Ophthalmol. 2010;47:160–7.
Cunha-Vaz JG, Travassos A. Breakdown of the blood–retinal barriers and cystoid macular edema. Surv Ophthalmol. 1984;28:485–92.
Fishman GA, Cunha-Vaz J, Salzano T. Vitreous fluorophotometry in patients with retinitis pigmentosa. Arch Ophthalmol. 1981;99:1202–7.
Kuchle M, Nguyen NX, Martus P, Freissler K, Schalnus R. Aqueous flare in retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol. 1998;236:426–33.
Vinores SA, Kuchle M, Derevjanik NL, Henderer JD, Mahlow J, Green WR, et al. Blood-retinal barrier breakdown in retinitis pigmentosa: light and electron microscopic immunolocalization. Histol Histopathol. 1995;10:913–23.
Cox SN, Hay E, Bird AC. Treatment of chronic macular edema with acetazolamide. Arch Ophthalmol. 1988;106:1190–5.
Fishman GA, Fishman M, Maggiano J. Macular lesions associated with retinitis pigmentosa. Arch Ophthalmol. 1977;95:798–803.
Makiyama Y, Oishi A, Otani A, Ogino K, Nakagawa S, Kurimoto M, et al. Prevalence and spatial distribution of cystoid spaces in retinitis pigmentosa: investigation with spectral domain optical coherence tomography. Retina. 2014;34:981–8.
Acknowledgments
We thank Hitomi Suetsugu for technical assistance and the Center for Anatomical, Pathological, and Forensic Medical Research for preparation of the histological sections. This study was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Grand-in-Aid for Young Scientists No. 24791850 and for the Innovative Techno-Hub for Integrated Medical Bio-Imaging of the Project for Developing Innovation Systems No. 24249082). This study was also supported by grants from the Ministry of Health, Labour and Welfare of Japan and Japan Society for the Promotion of Science.
Conflicts of interest
T. Hasegawa, none; H.O. Ikeda, Grant (the Astellas Foundation for Research on Metabolic Disorders, the Japan Foundation for Applied Enzymology, the Uehara Memorial Foundation, YOKOYAMA Foundation for Clinical Pharmacology, Mochida Memorial Foundation, Japan Intractable Diseases Research Foundation, Japan Research Foundation for Clinical Pharmacology); N. Nakano, none; Y. Muraoka, none; T. Tsuruyama, None; K. O. Furuta, none; H. Kohda, none; N. Yoshimura, grant (Nidek, Topcon, Canon, Novartis Japan, Bayer, Santen, Senju), honorary (Nidek, Canon, Novartis Japan, Bayer, Santen, Senju), and consultant fee (Nidek, Canon).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Hasegawa, T., Ikeda, H.O., Nakano, N. et al. Changes in morphology and visual function over time in mouse models of retinal degeneration: an SD-OCT, histology, and electroretinography study. Jpn J Ophthalmol 60, 111–125 (2016). https://doi.org/10.1007/s10384-015-0422-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-015-0422-0