Summary
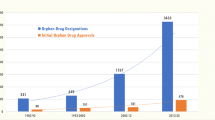
Since 1983 more than 300 drugs have been developed and approved for orphan diseases. However, considering the development of novel diagnosis tools, the number of rare diseases vastly outpaces therapeutic discovery. Academic centers and nonprofit institutes are now at the forefront of rare disease R&D, partnering with pharmaceutical companies when academic researchers discover novel drugs or targets for specific diseases, thus reducing the failure risk and cost for pharmaceutical companies. Considerable progress has occurred in the art of orphan drug discovery, and a symbiotic relationship now exists between pharmaceutical industry, academia, and philanthropists that provides a useful framework for orphan disease therapeutic discovery. Here, the current state-of-the-art of drug discovery for orphan diseases is reviewed. Current technological approaches and challenges for drug discovery are considered, some of which can present somewhat unique challenges and opportunities in orphan diseases, including the potential for personalized medicine, gene therapy, and phenotypic screening.
Zusammenfassung
Seit 1983 sind mehr als 300 Medikamente für seltene Krankheiten entwickelt und zugelassen worden. In Anbetracht dessen aber, wie rasant sich einerseits neue Diagnostik-Werkzeuge entwickeln und andererseits die Anzahl seltener Krankheiten zunimmt, hinkt die Arzneientwicklung hinterher. Wissenschaftliche Zentren und gemeinnützige Organisationen stehen nun an der Spitze der Forschung und Entwicklung für seltene Krankheiten. Sie arbeiten mit Pharmakonzernen zusammen, wenn akademische Forscher neue Medikamente oder Targets für einzelne Krankheiten entdecken, und reduzieren somit das Risiko des Misslingens als auch die Kosten für besagte Konzerne. Es hat beträchtliche Fortschritte auf dem Gebiet der Pharmaforschung für seltene Krankheiten gegeben, und es besteht inzwischen eine Art symbiotischer Beziehung zwischen der Pharmaindustrie, der akademischen Welt und Philanthropen, welche ein nützliches Gerüst für die Entwicklung neuer Arzneimittel für seltene Krankheiten bietet. Die vorliegende Arbeit fasst den aktuellen Stand der Pharmaforschung für seltene Krankheiten zusammen. Ferner beleuchtet sie die aktuellen technologischen Ansätze und Hürden der Arzneimittelentwicklung, die zum Teil einzigartige Chancen und Herausforderungen mit sich bringen, einschließlich potentieller Verwendungen in Zusammenhang mit personalisierter Medizin, Gentherapie und phänotypischem Screening.
Similar content being viewed by others
References
Cuatrecasas P. Drug discovery in jeopardy. J Clin Invest. 2006;116:2837–42.
Wästfelt M, Fadeel B, Henter J-I. A journey of hope: lessons learned from studies on rare diseases and orphan drugs. J Intern Med. 2006;260:1–10.
Haffner ME, Whitley J, Moses M. Two decades of orphan product development. Nat Rev Drug Discov. 2002;1:821–5.
Dolgin E. Big pharma moves from “blockbusters” to “niche busters”. Nat Med. 2010;16:837–7.
Dunne S, Shannon B, Dunne C, Cullen W. A review of the differences and similarities between generic drugs and their originator counterparts, including economic benefits associated with usage of generic medicines, using Ireland as a case study. BMC Pharmacol Toxicol. 2013;14:1.
Haffner ME, Torrent-Farnell J, Maher PD. Does orphan drug legislation really answer the needs of patients? Lancet. 2008;371:2041–4.
Search Orphan Drug Designations and Approvals. http://www.accessdata.fda.gov/scripts/opdlisting/oopd/index.cfm. Accessed 13 April 2015.
Hershfield MS, Buckley RH, Greenberg ML, Melton AL, Schiff R, Hatem C, et al. Treatment of adenosine deaminase deficiency with polyethylene glycol–modified adenosine deaminase. N Engl J Med. 1987;316:589–96.
Scott CR. The genetic tyrosinemias. Am J Med Genet C Semin. Med Genet. 2006;142C:121–6.
The Office of Rare Diseases Research (ORDR). http://rarediseases.info.nih.gov/aboutus.aspx. Accessed 9 April 2015.
Brewer GJ. Drug development for orphan diseases in the context of personalized medicine. Transl Res 2009;154:314–22.
Futerman AH, van Meer G. The cell biology of lysosomal storage disorders. Nat Rev Mol Cell Biol. 2004;5:554–65.
Platt FM, Boland B, van der Spoel AC. Lysosomal storage disorders: the cellular impact of lysosomal dysfunction. J Cell Biol. 2012;199:723–34.
Parenti G, Andria G, Ballabio A. Lysosomal storage diseases: from pathophysiology to therapy. Annu Rev Med. 2015;66:471–86.
Xu K, Coté TR. Database identifies FDA-approved drugs with potential to be repurposed for treatment of orphan diseases. Brief Bioinform. 2011. doi:10.1093/bib/bbr006.
Parenti G. Treating lysosomal storage diseases with pharmacological chaperones: from concept to clinics. EMBO Mol Med. 2009;1:268–79.
Parenti G, Andria G, Valenzano KJ. Pharmacological chaperone therapy: preclinical development, clinical translation, and prospects for the treatment of lysosomal storage disorders. Mol Ther. http://www.nature.com/mt/journal/vaop/ncurrent/full/mt201562a.html. Accessed 26 May 2015.
Janovick JA, Brothers SP, Cornea A, Bush E, Goulet MT, Ashton WT, et al. Refolding of misfolded mutant GPCR: post-translational pharmacoperone action in vitro. Mol Cell Endocrinol. 2007;272:77–85.
Hanrahan JW, Sampson HM, Thomas DY. Novel pharmacological strategies to treat cystic fibrosis. Trends Pharmacol Sci. 2013;34:119–25.
Wang G-N, Reinkensmeier G, Zhang S-W, Zhou J, Zhang L-R, Zhang L-H, et al. Rational design and synthesis of highly potent pharmacological chaperones for treatment of N370S mutant Gaucher disease. J Med Chem. 2009;52:3146–9.
Brothers SP, Janovick JA, Conn PM. Calnexin regulated gonadotropin-releasing hormone receptor plasma membrane expression. J Mol Endocrinol. 2006;37:479–88.
Swinney DC, Anthony J. How were new medicines discovered? Nat Rev Drug Discov. 2011;10:507–19.
Moffat JG, Rudolph J, Bailey D. Phenotypic screening in cancer drug discovery—past, present and future. Nat Rev Drug Discov. 2014;13:588–602.
Marks PA, Breslow R. Dimethyl sulfoxide to vorinostat: development of this histone deacetylase inhibitor as an anticancer drug. Nat Biotechnol. 2007;25:84–90.
Ferlini A, Scotton C, Novelli G. Biomarkers in rare diseases. Public Health Genomics. 2013;16:313–21.
Carey JC. The importance of case reports in advancing scientific knowledge of rare diseases. In: Paz MP de la, Groft SC, editors. Rare Dis Epidemiol. Springer Netherlands; 2010. pp. 77–86. http://link.springer.com/chapter/10.1007/978-90-481-9485-85. Accessed 26 May 2015.
Kesselheim AS, Gagne JJ. Strategies for postmarketing surveillance of drugs for rare diseases. Clin Pharmacol Ther. 2014;95:265–8.
US Department of Health and Human Services Food and Drug Administration. Center for drug evaluation and research. Guidance for Industry, Investigators and Reviewers Exploratory IND Studies- UCM078933.pdf. Guid Ind Investig Rev Explor IND Stud. 2006. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM078933.pdf. Accessed 31 Oct 2015.
Griggs RC, Batshaw M, Dunkle M, Gopal-Srivastava R, Kaye E, Krischer J, et al. Clinical research for rare disease: opportunities, challenges, and solutions. Mol Genet Metab. 2009;96:20–6.
Volmar C-H, Brothers S, Wahlestedt C. Development of personalized small molecule modulator screening strategies: upregulation of alpha-L-iduronidase in Mucopolysaccharidosis Type I (MPSI) patient cells. Neuropsychopharmacology. 2012;38:S79–197 (M96).
Avila AM, Burnett BG, Taye AA, Gabanella F, Knight MA, Hartenstein P, et al. Trichostatin A increases SMN expression and survival in a mouse model of spinal muscular atrophy. J Clin Invest. 2007;117:659–71.
Home | Orphan Disease Center | University of Pennsylvania | Perelman School of Medicine. http://www.med.upenn.edu/orphandisease/index.shtml. Accessed 28 May 2015.
The Center for Rare and Neglected Diseases at the University of Notre Dame. http://www3.nd.edu/~crnd/about_page.htm. Accessed 28 May 2015.
Manton Center for Orphan Disease Research | Research + Innovation | Boston Children’s Hospital. http://www.childrenshospital.org/research-and-innovation/research/centers/manton-center-for-orphan-disease-research. Accessed 28 May 2015.
Center For Orphan Drug Research—College of Pharmacy—University of Minnesota. http://www.pharmacy.umn.edu/codr/. Accessed 28 May 2015.
The Institute—TIGEM. http://www.tigem.it/the-institute. Accessed 28 May 2015.
Center for Therapeutic Innovation | Research at Miller School of Medicine. http://psychiatry.med.miami.edu/research/center-for-therapeutic-innovation. Accessed 28 May 2015.
KGI | Center for Rare Disease Therapies. http://www.kgi.edu/faculty-and-research/kgi-centers/center-for-rare-disease-therapies.html. Accessed 28 May 2015.
Leukodystrophies Research | Kennedy Krieger Institute. http://www.kennedykrieger.org/patient-care/patient-care-centers/center-for-leukodystrophies/research-program. Accessed 28 May 2015.
Coles LD, Cloyd JC. The role of academic institutions in the development of drugs for rare and neglected diseases. Clin Pharmacol Ther. 2012;92:193–202.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
C.-H. Volmar, C. Wahlestedt, and S. P. Brothers declare that there are no actual or potential conflicts of interest in relation to this article.
Rights and permissions
About this article
Cite this article
Volmar, CH., Wahlestedt, C. & Brothers, S. Orphan diseases: state of the drug discovery art. Wien Med Wochenschr 167, 197–204 (2017). https://doi.org/10.1007/s10354-015-0423-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10354-015-0423-0