Summary
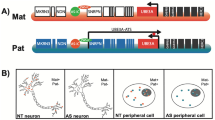
Angelman syndrome (AS) is a severe neurodevelopmental disorder caused by a loss of the maternally inherited UBE3A; the paternal UBE3A is silenced in neurons by a mechanism involving an antisense transcript (UBE3A-AS). We reviewed the published information on clinical trials that have been completed as well as the publicly available information on ongoing trials of therapies for AS. Attempts at hypermethylating the maternal locus through dietary compounds were ineffective. The results of a clinical trial using minocycline as a matrix metalloproteinase-9 inhibitor were inconclusive; another clinical trial is underway. Findings from a clinical trial using L-dopa to alter phosphorylation of calcium/calmodulin-dependent kinase II are awaited. Topoisomerase inhibitors and antisense oligonucleotides are being developed to directly inhibit UBE3A-AS. Other strategies targeting specific pathways are briefly discussed. We also reviewed the medications that are currently used to treat seizures and sleep disturbances, which are two of the more debilitating manifestations of AS.
Zusammenfassung
Das Angelman-Syndrom (AS) ist eine schwerwiegende neurologische Entwicklungsstörung, die durch die fehlende Expression des maternal vererbten UBE3A-Gens verursacht wird; das paternale UBE3A wird in Neuronen durch einen Mechanismus stillgelegt, der ein Antisense-Transkript (UBE3A-AS) involviert. Publizierte Daten zu abgeschlossenen klinischen Studien sowie vorliegende Informationen aus laufenden Studien zur AS-Therapie wurden überprüft. Versuche, den maternalen Genort mittels diätetischer Präparate zu hypermethylieren, sind wirkungslos geblieben. Als inkonklusiv erwiesen sich die Ergebnisse einer klinischen Studie, die Minocyclin zur Hemmung der Matrix-Metalloprotease-9 einsetzte; eine weitere klinische Studie ist in Ausarbeitung. Die Ergebnisse einer klinischen Untersuchung zur Änderung der Phosphorylierung der Calcium/Calmodulin-abhängigen Kinase II durch Levodopa werden erwartet. Topoisomerasehemmer und Antisense-Oligonukleotide werden zur direkten UBE3A-AS-Hemmung entwickelt. Andere Strategien zur Targetierung spezifischer Leitungsbahnen werden kurz besprochen. Die heute eingesetzten medikamentösen Therapien bei einigen der schwerwiegendsten AS-Manifestationen – Insulten und Dyssomnien – wurden gesichtet.

Similar content being viewed by others
References
Thibert RL, Larson AM, Hsieh DT, et al. Neurologic manifestations of Angelman syndrome. Pediatr Neurol. 2013;48:271–9.
Bird LM. Angelman syndrome: review of clinical and molecular aspects. Appl Clin Genet. 2014;7:93–104.
Larson AM, Shinnick JE, Shaaya EA, et al. Angelman syndrome in adulthood. Am J Med Genet A. 2015;167 A:331–44.
Williams CA, Beaudet AL, Clayton-Smith J, et al. Angelman syndrome 2005: updated consensus for diagnostic criteria. Am J Med Genet A. 2006;140:413–8.
Dagli A, Buiting K, Williams CA. Molecular and clinical aspects of Angelman syndrome. Mol Syndromol. 2012;2:100–12.
Low D, Chen KS. Genome-wide gene expression profiling of the Angelman syndrome mice with Ube3a mutation. Eur J Hum Genet. 2010;18:1228–35.
Jensen L, Farook MF, Reiter LT. Proteomic profiling in Drosophila reveals potential Dube3a regulation of the actin cytoskeleton and neuronal homeostasis. PLoS One. 2013;8:e61952.
Buiting K, Lich C, Cottrell S, et al. A 5-kb imprinting center deletion in a family with Angelman syndrome reduces the shortest region of deletion overlap to 880 bp. Hum Genet. 1999;105:665–6.
Dubose AJ, Smith EY, Yang TP, et al. A new deletion refines the boundaries of the murine Prader–Willi syndrome imprinting center. Hum Mol Genet. 2011;20:3461–6.
Dittrich B, Buiting K, Korn B, et al. Imprint switching on human chromosome 15 may involve alternative transcripts of the SNRPN gene. Nat Genet. 1996;14:163–70.
Farber C, Dittrich B, Buiting K, et al. The chromosome 15 imprinting centre (IC) region has undergone multiple duplication events and contains an upstream exon of SNRPN that is deleted in all Angelman syndrome patients with an IC microdeletion. Hum Mol Genet. 1999;8:337–43.
Lewis MW, Brant JO, Kramer JM, et al. Angelman syndrome imprinting center encodes a transcriptional promoter. Proc Natl Acad Sci USA. 2015;112:6871–5.
Chamberlain SJ. RNAs of the human chromosome 15q11-q13 imprinted region. Wiley Interdiscip Rev RNA. 2013;4:155–66.
Chamberlain SJ, Brannan CI. The Prader–Willi syndrome imprinting center activates the paternally expressed murine Ube3a antisense transcript but represses paternal Ube3a. Genomics. 2001;73:316–22.
Dindot SV, Antalffy BA, Bhattacharjee MB, et al. The Angelman syndrome ubiquitin ligase localizes to the synapse and nucleus, and maternal deficiency results in abnormal dendritic spine morphology. Hum Mol Genet. 2008;17:111–8.
Jiang YH, Armstrong D, Albrecht U, et al. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron. 1998;21:799–811.
Yashiro K, Riday TT, Condon KH, et al. Ube3a is required for experience-dependent maturation of the neocortex. Nat Neurosci. 2009;12:777–83.
Dizik M, Christman JK, Wainfan E. Alterations in expression and methylation of specific genes in livers of rats fed a cancer promoting methyl-deficient diet. Carcinogenesis. 1991;12:1307–12.
Van den Veyver IB. Genetic effects of methylation diets. Annu Rev Nutr. 2002;22:255–82.
Peters SU, Bird LM, Kimonis V, et al. Double-blind therapeutic trial in Angelman syndrome using betaine and folic acid. Am J Med Genet A. 2010;152 A:1994–2001.
Bird LM, Tan WH, Bacino CA, et al. A therapeutic trial of pro-methylation dietary supplements in Angelman syndrome. Am J Med Genet A. 2011;155 A:2956–63.
Huang HS, Allen JA, Mabb AM, et al. Topoisomerase inhibitors unsilence the dormant allele of Ube3a in neurons. Nature. 2012;481:185–9.
Powell WT, Coulson RL, Gonzales ML, et al. R-loop formation at Snord116 mediates topotecan inhibition of Ube3a-antisense and allele-specific chromatin decondensation. Proc Natl Acad Sci U S A. 2013;110:13938–43.
Skourti-Stathaki K, Proudfoot NJ. A double-edged sword: r loops as threats to genome integrity and powerful regulators of gene expression. Genes Dev. 2014;28:1384–96.
King IF, Yandava CN, Mabb AM, et al. Topoisomerases facilitate transcription of long genes linked to autism. Nature. 2013;501:58–62.
Plasschaert RN, Bartolomei MS. Autism: a long genetic explanation. Nature. 2013;501:36–7.
Meng L, Person RE, Huang W, et al. Truncation of Ube3a-ATS unsilences paternal Ube3a and ameliorates behavioral defects in the Angelman syndrome mouse model. PLoS Genet. 2013;9:e1004039.
Santos RD, Raal FJ, Catapano AL, et al. Mipomersen, an antisense oligonucleotide to apolipoprotein B-100, reduces lipoprotein(a) in various populations with hypercholesterolemia: results of 4 phase III trials. Arterioscler Thromb Vasc Biol. 2015;35:689–99.
Marafini I, Di Fusco D, Calabrese E, et al. Antisense approach to inflammatory bowel disease: prospects and challenges. Drugs. 2015;75:723–30.
Voit T, Topaloglu H, Straub V, et al. Safety and efficacy of drisapersen for the treatment of Duchenne muscular dystrophy (DEMAND II): an exploratory, randomised, placebo-controlled phase 2 study. Lancet Neurol. 2014;13:987–96.
Evers MM, Toonen LJ, van Roon-Mom WM. Antisense oligonucleotides in therapy for neurodegenerative disorders. Adv Drug Deliv Rev. 2015.
Meng L, Ward AJ, Chun S, et al. Towards a therapy for Angelman syndrome by targeting a long non-coding RNA. Nature. 2015;518:409–12.
Daily JL, Nash K, Jinwal U, et al. Adeno-associated virus-mediated rescue of the cognitive defects in a mouse model for Angelman syndrome. PLoS One. 2011;6:e27221.
Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–90.
Blitzer RD, Iyengar R, Landau EM. Postsynaptic signaling networks: cellular cogwheels underlying long-term plasticity. Biol Psychiatry. 2005;57:113–9.
Giese KP, Fedorov NB, Filipkowski RK, et al. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–3.
Elgersma Y, Fedorov NB, Ikonen S, et al. Inhibitory autophosphorylation of CaMKII controls PSD association, plasticity, and learning. Neuron. 2002;36:493–505.
Weeber EJ, Jiang YH, Elgersma Y, et al. Derangements of hippocampal calcium/calmodulin-dependent protein kinase II in a mouse model for Angelman mental retardation syndrome. J Neurosci. 2003;23:2634–44.
van Woerden GM, Harris KD, Hojjati MR, et al. Rescue of neurological deficits in a mouse model for Angelman syndrome by reduction of alphaCaMKII inhibitory phosphorylation. Nat Neurosci. 2007;10:280–2.
Brown AM, Deutch AY, Colbran RJ. Dopamine depletion alters phosphorylation of striatal proteins in a model of Parkinsonism. Eur J Neurosci. 2005;22:247–56.
Mulherkar SA, Jana NR. Loss of dopaminergic neurons and resulting behavioural deficits in mouse model of Angelman syndrome. Neurobiol Dis. 2010;40:586–92.
Riday TT, Dankoski EC, Krouse MC, et al. Pathway-specific dopaminergic deficits in a mouse model of Angelman syndrome. J Clin Invest. 2012;122:4544–54.
Huntley GW. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat Rev Neurosci. 2012;13:743–57.
Dziembowska M, Wlodarczyk J. MMP9: a novel function in synaptic plasticity. Int J Biochem Cell Biol. 2012;44:709–13.
Fragkouli A, Papatheodoropoulos C, Georgopoulos S, et al. Enhanced neuronal plasticity and elevated endogenous sAPPalpha levels in mice over-expressing MMP9. J Neurochem. 2012;121:239–51.
Bilousova TV, Dansie L, Ngo M, et al. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J Med Genet. 2009;46:94–102.
Iulita MF, Do Carmo S, Ower AK, et al. Nerve growth factor metabolic dysfunction in Down̕s syndrome brains. Brain. 2014;137:860–72.
Sidhu H, Dansie LE, Hickmott PW, et al. Genetic removal of matrix metalloproteinase 9 rescues the symptoms of fragile X syndrome in a mouse model. J Neurosci. 2014;34:9867–79.
Cullen SI, Cohan RH. Minocycline therapy in acne vulgaris. Cutis. 1976;17:1208–10, 1214.
Jonas M, Cunha BA. Minocycline. Ther Drug Monit. 1982;4:137–45.
Griffin MO, Fricovsky E, Ceballos G, et al. Tetracyclines: a pleitropic family of compounds with promising therapeutic properties. Review of the literature. Am J Physiol Cell Physiol. 2010;299:C539–48.
Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4:28–37.
Leigh MJ, Nguyen DV, Mu Y, et al. A randomized double-blind, placebo-controlled trial of minocycline in children and adolescents with fragile X syndrome. J Dev Behav Pediatr. 2013;34:147–55.
Dziembowska M, Pretto DI, Janusz A, et al. High MMP-9 activity levels in fragile X syndrome are lowered by minocycline. Am J Med Genet A. 2013;161 A:1897–903.
Grieco JC, Ciarlone SL, Gieron-Korthals M, et al. An open-label pilot trial of minocycline in children as a treatment for Angelman syndrome. BMC Neurol. 2014;14:232.
Greer PL, Hanayama R, Bloodgood BL, et al. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell. 2010;140:704–16.
Margolis SS, Salogiannis J, Lipton DM, et al. EphB-mediated degradation of the RhoA GEF Ephexin5 relieves a developmental brake on excitatory synapse formation. Cell. 2010;143:442–55.
Kuhnle S, Mothes B, Matentzoglu K, et al. Role of the ubiquitin ligase E6AP/UBE3A in controlling levels of the synaptic protein Arc. Proc Natl Acad Sci U S A. 2013;110:8888–93.
Mandel-Brehm C, Salogiannis J, Dhamne SC, et al. Seizure-like activity in a juvenile Angelman syndrome mouse model is attenuated by reducing Arc expression. Proc Natl Acad Sci USA. 2015;112:5129–34.
Roden WH, Peugh LD, Jansen LA. Altered GABA(A) receptor subunit expression and pharmacology in human Angelman syndrome cortex. Neurosci Lett. 2010;483:167–72.
Egawa K, Kitagawa K, Inoue K, et al. Decreased tonic inhibition in cerebellar granule cells causes motor dysfunction in a mouse model of Angelman syndrome. Sci Transl Med. 2012;4:163ra57.
Baudry M, Bi X, Gall C, et al. The biochemistry of memory: the 26 year journey of a ‘new and specific hypothesis̕’. Neurobiol Learn Mem. 2011;95:125–33.
Lynch G, Rex CS, Chen LY, et al. The substrates of memory: defects, treatments, and enhancement. Eur J Pharmacol. 2008;585:2–13.
Simmons DA, Rex CS, Palmer L, et al. Up-regulating BDNF with an ampakine rescues synaptic plasticity and memory in Huntington̕s disease knockin mice. Proc Natl Acad Sci USA. 2009;106:4906–11.
Baudry M, Kramar E, Xu X, et al. Ampakines promote spine actin polymerization, long-term potentiation, and learning in a mouse model of Angelman syndrome. Neurobiol Dis. 2012;47:210–5.
Chang PK, Verbich D, McKinney RA. AMPA receptors as drug targets in neurological disease–advantages, caveats, and future outlook. Eur J Neurosci. 2012;35:1908–16.
Panja D, Bramham CR. BDNF mechanisms in late LTP formation: a synthesis and breakdown. Neuropharmacology. 2014;76 Pt C:664–76.
Cao C, Rioult-Pedotti MS, Migani P, et al. Impairment of TrkB-PSD-95 signaling in Angelman syndrome. PLoS Biol. 2013;11:e1001478.
Yoshii A, Constantine-Paton M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat Neurosci. 2007;10:702–11.
Yoshii A, Murata Y, Kim J, et al. TrkB and protein kinase Mzeta regulate synaptic localization of PSD-95 in developing cortex. J Neurosci. 2011;31:11894–904.
Kaphzan H, Hernandez P, Jung JI, et al. Reversal of impaired hippocampal long-term potentiation and contextual fear memory deficits in Angelman syndrome model mice by ErbB inhibitors. Biol Psychiatry. 2012;72:182–90.
Kwon OB, Longart M, Vullhorst D, et al. Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J Neurosci. 2005;25:9378–83.
Pitcher GM, Beggs S, Woo RS, et al. ErbB4 is a suppressor of long-term potentiation in the adult hippocampus. Neuroreport. 2008;19:139–43.
Sun J, Liu Y, Moreno S, et al. Imbalanced mechanistic target of rapamycin C1 and C2 activity in the cerebellum of Angelman syndrome mice impairs motor function. J Neurosci. 2015;35:4706–18.
Rogers JT, Rusiana I, Trotter J, et al. Reelin supplementation enhances cognitive ability, synaptic plasticity, and dendritic spine density. Learn Mem. 2011;18:558–64.
Hethorn WR, Ciarlone SL, Filonova I, et al. Reelin supplementation recovers synaptic plasticity and cognitive deficits in a mouse model for Angelman syndrome. Eur J Neurosci. 2015;41:1372–80.
Su H, Fan W, Coskun PE, et al. Mitochondrial dysfunction in CA1 hippocampal neurons of the UBE3A deficient mouse model for Angelman syndrome. Neurosci Lett. 2011;487:129–33.
Llewellyn KJ, Nalbandian A, Gomez A, et al. Administration of CoQ10 analogue ameliorates dysfunction of the mitochondrial respiratory chain in a mouse model of Angelman syndrome. Neurobiol Dis. 2015;76:77–86.
Pelc K, Boyd SG, Cheron G, et al. Epilepsy in Angelman syndrome. Seizure. 2008;17:211–7.
Thibert RL, Conant KD, Braun EK, et al. Epilepsy in Angelman syndrome: a questionnaire-based assessment of the natural history and current treatment options. Epilepsia. 2009;50:2369–76.
Valente KD, Varela MC, Koiffmann CP, et al. Angelman syndrome caused by deletion: a genotype-phenotype correlation determined by breakpoint. Epilepsy Res. 2013;105:234–9.
Dion MH, Novotny EJ Jr, Carmant L, et al. Lamotrigine therapy of epilepsy with Angelman̕s syndrome. Epilepsia. 2007;48:593–6.
Franz DN, Glauser TA, Tudor C, et al. Topiramate therapy of epilepsy associated with Angelman̕s syndrome. Neurology. 2000;54:1185–8.
Ostergaard JR, Balslev T. Efficacy of different antiepileptic drugs in children with Angelman syndrome associated with 15q11-13 deletion: the Danish experience. Dev Med Child Neurol. 2001;43:718–9.
Valente KD, Koiffmann CP, Fridman C, et al. Epilepsy in patients with Angelman syndrome caused by deletion of the chromosome 15q11-13. Arch Neurol. 2006;63:122–8.
Neal EG, Chaffe H, Schwartz RH, et al. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia. 2009;50:1109–17.
Neal EG, Chaffe H, Schwartz RH, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7:500–6.
Levy RG, Cooper PN, Giri P. Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst Rev. 2012;3:CD001903.
Evangeliou A, Doulioglou V, Haidopoulou K, et al. Ketogenic diet in a patient with Angelman syndrome. Pediatr Int. 2010;52:831–4.
Stein D, Chetty M, Rho JM. A “happy” toddler presenting with sudden, life-threatening seizures. Semin Pediatr Neurol. 2010;17:35–8.
Kossoff EH, Zupec-Kania BA, Amark PE, et al. Optimal clinical management of children receiving the ketogenic diet: recommendations of the International Ketogenic Diet Study Group. Epilepsia. 2009;50:304–17.
Bergqvist AG. Long-term monitoring of the ketogenic diet: do’s and Don’ts. Epilepsy Res. 2012;100:261–6.
Hemingway C, Freeman JM, Pillas DJ, et al. The ketogenic diet: a 3- to 6-year follow-up of 150 children enrolled prospectively. Pediatrics. 2001;108:898–905.
Thibert RL, Pfeifer HH, Larson AM, et al. Low glycemic index treatment for seizures in Angelman syndrome. Epilepsia. 2012;53:1498–502.
Forrest KM, Young H, Dale RC, et al. Benefit of corticosteroid therapy in Angelman syndrome. J Child Neurol. 2009;24:952–8.
dos Santos RG, Hallak JE, Leite JP, et al. Phytocannabinoids and epilepsy. J Clin Pharm Ther. 2015;40:135–43.
Didden R, Sigafoos J. A review of the nature and treatment of sleep disorders in individuals with developmental disabilities. Res Dev Disabil. 2001;22:255–72.
Goldman SE, Bichell TJ, Surdyka K, et al. Sleep in children and adolescents with Angelman syndrome: association with parent sleep and stress. J Intellect Disabil Res. 2012;56:600–8.
Bruni O, Ferri R, D’Agostino G, et al. Sleep disturbances in Angelman syndrome: a questionnaire study. Brain Dev. 2004;26:233–40.
Didden R, Korzilius H, Smits MG, et al. Sleep problems in individuals with Angelman syndrome. Am J Ment Retard. 2004;109:275–84.
Walz NC, Beebe D, Byars K. Sleep in individuals with Angelman syndrome: parent perceptions of patterns and problems. Am J Ment Retard. 2005;110:243–52.
Miano S, Bruni O, Leuzzi V, et al. Sleep polygraphy in Angelman syndrome. Clin Neurophysiol. 2004;115:938–45.
Miano S, Bruni O, Elia M, et al. Sleep breathing and periodic leg movement pattern in Angelman Syndrome: a polysomnographic study. Clin Neurophysiol. 2005;116:2685–92.
Pelc K, Cheron G, Boyd SG, et al. Are there distinctive sleep problems in Angelman syndrome? Sleep Med. 2008;9:434–41.
Takaesu Y, Komada Y, Inoue Y. Melatonin profile and its relation to circadian rhythm sleep disorders in Angelman syndrome patients. Sleep Med. 2012;13:1164–70.
Zhdanova IV, Wurtman RJ, Wagstaff J. Effects of a low dose of melatonin on sleep in children with Angelman syndrome. J Pediatr Endocrinol Metab. 1999;12:57–67.
Braam W, Didden R, Smits MG, et al. Melatonin for chronic insomnia in Angelman syndrome: a randomized placebo-controlled trial. J Child Neurol. 2008;23:649–54.
Braam W, Smits MG, Didden R, et al. Exogenous melatonin for sleep problems in individuals with intellectual disability: a meta-analysis. Dev Med Child Neurol. 2009;51:340–9.
Schwichtenberg AJ, Malow BA. Melatonin treatment in children with developmental disabilities. Sleep Med Clin. 2015;10:181–7.
Ingrassia A, Turk J. The use of clonidine for severe and intractable sleep problems in children with neurodevelopmental disorders–a case series. Eur Child Adolesc Psychiatry. 2005;14:34–40.
Allen KD, Kuhn BR, DeHaai KA, et al. Evaluation of a behavioral treatment package to reduce sleep problems in children with Angelman Syndrome. Res Dev Disabil. 2013;34:676–86.
Grigg-Damberger M, Ralls F. Treatment strategies for complex behavioral insomnia in children with neurodevelopmental disorders. Curr Opin Pulm Med. 2013;19:616–25.
Conant KD, Thibert RL, Thiele EA. Epilepsy and the sleep-wake patterns found in Angelman syndrome. Epilepsia. 2009;50:2497–500.
Jana NR. Understanding the pathogenesis of Angelman syndrome through animal models. Neural Plast. 2012;2012:710943.
Miura K, Kishino T, Li E, et al. Neurobehavioral and electroencephalographic abnormalities in Ube3a maternal-deficient mice. Neurobiol Dis. 2002;9:149–59.
Gatto CL, Broadie K. Drosophila modeling of heritable neurodevelopmental disorders. Curr Opin Neurobiol. 2011;21:834–41.
Huang HS, Burns AJ, Nonneman RJ, et al. Behavioral deficits in an Angelman syndrome model: effects of genetic background and age. Behav Brain Res. 2013;243:79–90.
Acknowledgements
The authors would like to thank Stormy J. Chamberlain, Ph.D. (University of Connecticut Health Center) for reviewing and revising the sections in this manuscript that describe imprinting in Angelman syndrome.
Conflict of interest
Wen-Hann Tan and Lynne Bird declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, WH., Bird, L.M. Pharmacological therapies for Angelman syndrome. Wien Med Wochenschr 167, 205–218 (2017). https://doi.org/10.1007/s10354-015-0408-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10354-015-0408-z