Abstract
The effect of various constant temperatures on survival, development, and adult longevity of Venturia canescens Gravenhorst (Hymenoptera: Ichneumonidae) parasitizing larvae of Plodia interpunctella Hübner (Lepidoptera: Pyralidae) was studied under laboratory conditions. The following temperatures were tested: 15, 17.5, 20, 22.5, 25, 27.5, 30, and 32.5°C. The percentage of parasitoids that completed development at each temperature as well as the days needed for the emergence of the parasitoid’s pupa and adult eclosion was measured. Adult longevity was estimated under the same conditions. Survival of V. canescens was significantly higher at 25 and 27.5°C compared to 17.5, 20, 30, and 32.5°C. No individual of V. canescens managed to complete development at 15°C. Overall, developmental time decreased significantly with increasing temperature within the range of 17.5–27.5°C. The lowest developmental time was observed at 27.5°C while the highest at 17.5°C. Upper and lower threshold temperatures for total development were estimated at 36.2 and 13.2°C, respectively. Optimum temperature for development and thermal constant were 30.6°C and 312.5 degree days, respectively. Adult longevity was also affected by temperature, as it was significantly reduced at higher temperatures compared to the lower ones. This information would be useful in determining the potential of using V. canescens as a biological agent in IPM programs, by optimizing mass rearing and release techniques of the parasitoid.
Similar content being viewed by others
Introduction
Insects are ectothermic organisms, meaning that their body temperature fluctuates with environmental temperature (Higley et al. 1986). Temperature is the major abiotic factor that has a strong influence on distribution, survival, abundance, fitness, and life history of insects (Cossins and Bowler 1987; Hallmann and Denlinger 1998). It is well known that temperature directly affects the rate of biochemical reactions and therefore has a strong influence on growth rate of insects (Davidowitz and Nijhout 2004). Insect development occurs within a specific temperature range (Speight et al. 1999). It is essential to understand how temperature affects survival, preadult development, and longevity, particularly for parasitoids, so that mass rearing and release techniques can be developed for use of suitable species as biocontrol agents of pests.
Venturia canescens Gravenhorst (Hymenoptera: Ichneumonidae) is a parthenogenetic solitary, koinobiont endoparasitoid of several pyralid moth larvae that are pests of stored products. Some of its hosts include Plodia interpunctella Hübner (Lepidoptera: Pyralidae), Ephestia kuehniella Zeller (Lepidoptera: Pyralidae), Ephestia elutella Hübner (Lepidoptera: Pyralidae), and Corcyra cephalonica Stainton (Lepidoptera: Pyralidae) (Salt 1975). Venturia canescens has been used as a model organism in numerous laboratory studies on insect physiology and biology, genetics, population dynamics, and insect behavior (Ahmed 1936; Rogers 1972; Salt 1975; Hubbard and Cook 1978; Bellows and Hassell 1988; Harvey et al. 1993; Begon et al. 1995; Driessen and Bernstein 1999; Harvey et al. 2001; Schneider et al. 2002; Eliopoulos et al. 2003; Thiel et al. 2006; Ozkan 2007; Lucchetta et al. 2008).
In the current study, the effect of various constant temperatures on survival to adulthood, developmental time, and adult longevity of V. canescens parasitizing fifth instar larvae of P. interpunctella is studied in the laboratory. Lower developmental threshold and thermal constant for each stage of development were calculated using a linear model (Campbell et al. 1974), while the upper developmental threshold and optimum temperature for development of V. canescens were estimated using a nonlinear model (Logan I) (Logan et al. 1976) fitted to the data.
Materials and methods
Insect cultures
Hosts and parasitoids were reared in laboratory conditions (25 ± 1°C, 60 ± 5% RH, 16:8 L:D). Plodia interpunctella larvae were cultured in clear plastic boxes (6 × 11 × 6 cm) and maintained on an artificial diet (Ashby et al. 1985), modified by the addition of 450 g dry pinto beans and 31 g agar (instead of 457.09 and 25.69 g agar, respectively). A colony of V. canescens was maintained on P. interpunctella as a host. Adults of V. canescens were cultured in clear plastic boxes (20 × 20 × 20 cm) provided with honey solution (10%) as food. Ten fifth instar larvae from the host colony were placed in each box together with 2 adult parasitoids. The following day the parasitized larvae were transferred individually to small plastic cups (3 cm in height and 4 cm in diameter) until adult eclosion. The procedure of parasitism in the colony was repeated every 2 days until the death of the adult parasitioids.
Both insects, the host and the parasitoid, originated from dried nuts collected from the Kavala region (41°01′Ν, 24°22′Ε) of northern Greece.
Effect of temperature on survival
Fifth instar larvae of P. interpunctella were placed into clear plastic boxes (20 × 20 × 20 cm), where young adults of V. canescens were reared (25 ± 1°C, 60 ± 5% RH), until parasitism occurred. Parasitism was easy to determine via a characteristic flexing or cocking of the abdomen of the wasp, which follows a successful oviposition, the “cocking” motion described by Rogers (1972). Parasitized larvae of P. interpunctella were removed and placed individually into small plastic cups (3 cm in height and 4 cm in diameter) provided with artificial diet, and afterward, they were transferred to incubators (Precision Scientific, General Electric, Louisville, KY) at eight constant temperatures (15, 17.5, 20, 22.5, 25, 27.5, 30, and 32.5°C) and 16:8 (L:D) photoperiod. Temperature in the incubators was monitored using internal thermometers. The variation in temperature in the incubators was ±0.2°C. Daily observations were made, and the percentage of parasitoids that completed successfully development until adulthood at each temperature regime was calculated. In some cases (15 and 17.5°C), the observations lasted up to 4 months, to confirm the death of the individual.
Effect of temperature on developmental time
Developmental time of V. canescens at the various constant temperatures was estimated from the same experiment. Daily observations were made to record the days needed for the emergence of the parasitoid’s pupa and adult eclosion at each temperature regime.
Lower developmental threshold
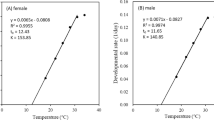
The relationship between the rate of development and temperature was based on the linear regression equation of the form y = a + bT, where y is the rate of development at temperature T and a and b are constants, which were estimated by least square regression. The lower developmental threshold (t) was calculated from t = −a/b, while the thermal constant (K) in degree days (DD) was estimated as K = 1/b (Campbell et al. 1974).
Standard errors (SE) for lower developmental threshold and thermal constant were calculated from equations of Campbell et al. (1974):
where y m is the sample mean, b is the linear equation parameter, s 2 is the residual mean square, and N is the sample size (number of temperatures tested).
The linear regressions included only the data for the linear portions of the relationship, up to the maximum developmental rate. The developmental times for 30 and 32.5°C were excluded because the relationship was clearly nonlinear above the temperature of 27.5 and would lead to unreasonable estimates of lower developmental threshold and day degrees.
Upper developmental threshold
Estimation of the upper developmental threshold was based on the Logan I nonlinear model (Logan et al. 1976). The equation of this model is of the form:
where d(T) is the rate of development at temperature T (°C), ψ is a directly measurable rate of temperature-dependent physiological process at some base temperature T b, ρ is a composite Q10 value for enzyme-catalyzed biochemical reactions, T m is the maximum temperature at which insect cannot survive for prolonged periods of time, and ΔΤ is a temperature range, above the optimum temperature for development and below T m.
The base temperature (T b) is either the minimum temperature used in the experiments or it can be hypothesized that T b = 0°C. In our experiments, the base temperature was considered to be 0°C for simplification of the model. In addition, the accuracy of the model is not affected by the hypothesis of base temperature being zero (Got et al. 1997).
Optimum temperature (T o) for development was calculated by the equation of Logan et al. (1976):
where \( \varepsilon = \frac{\Updelta T}{{T_{\rm m} }}\,{\text{and}}\,b_{\rm o} = \rho \times T_{\rm m}. \)
Effect of temperature on adult longevity
After eclosion, adult V. canescens individuals were removed from the incubators and placed into plastic cups (4.8 × 7.5 × 11.5 cm) separately. Individuals were then transferred back to the incubators of the eight constant temperatures they had emerged in. Honey solution (10%) was provided as food, and adult longevity was evaluated with daily observations, until the death of the parasitoid.
Statistical analysis
Analysis of variance (ANOVA) was carried out to test the effect of temperatures on developmental time and adult longevity of V. canescens (SPSS 2006). Means were compared using the Tukey’s-b test (a = 0.05) (Sokal and Rohlf 1995). Calculations of the linear model were performed with the statistical package SPSS Inc. (2006). The nonlinear regression was carried out with the statistical program JMP (SAS Institute 1989). Chi-square analysis was used to compare the survival of V. canescens at each temperature.
Results
Effect of temperature on survival
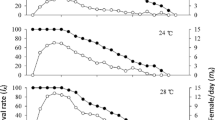
Survival of V. canescens was significantly higher at 25 and 27.5°C (83 and 80%, respectively) compared to 17.5, 20, 22.5, 30, and 32.5°C (8, 25, 35, 32, and 8%, respectively) while at 15°C no survival was observed (Fig. 1) (x 2 = 118.3; df = 6; P < 0.05).
Effect of temperature on developmental time
Regarding the developmental time (days needed from egg to adult) of V. canescens, the lowest growth rate was observed at 17.5°C, as 53.7 days were needed from parasitism to adult eclosion (Table 1) (F 6,125 = 211.56, P < 0.05). Developmental time decreased significantly as temperature rose within the range 17.5–27.5°C. Specifically, at 20°C, 44 days were needed from egg to adult eclosion, 34.2 days at 22.5°C and 27.4 days at 25°C. The shortest developmental time was observed at 27.5°C (20.8 days). At 30°C, developmental time of V. canescens increased significantly compared to 27.5°C, as 23 days were needed from egg to adult eclosion while at 32.5°C development took 21.8 days (Table 1).
Lower and upper developmental thresholds
Based on the linear model, the lower developmental thresholds (t) and degree day requirements (K) varied significantly with life stage (Table 2). The lower developmental threshold for the larvae of V. canescens was estimated at 13.2°C while the threshold for pupae was 12.8°C. The thermal constant for larval development was estimated at 158.7 DD while the constant for pupal development was estimated at 149.3 DD. The lower developmental threshold and the thermal constant for total development were calculated at 13.2°C and 312.5 DD, respectively.
The optimal temperature (T o) estimated for all life stages of V. canescens ranged between 28.8 and 30.6°C, whereas the upper developmental thresholds (T m) ranged between 34.2 and 36.2°C. The optimal temperature (T o) and the upper developmental threshold (T m) for pupae and total development were similar (Table 3).
Effect of temperature on adult longevity
Adult longevity was affected by temperature, as it was significantly reduced at 27.5°C (8.3 days) and 30°C (9.4 days) compared to 17.5°C (28.3 days), 20°C (22.5 days), 22.5°C (19.8 days), and 25°C (17.8 days). The lowest longevity was observed at 32.5°C (5.7 days) (Table 4) (F 6,125 = 15.124, P < 0.05).
Discussion
Temperature has a profound effect on distribution, colonization, survival, abundance, behavior, fitness, and life history traits of insects in general (Cossins and Bowler 1987; Hallmann and Denlinger 1998; Milonas and Savopoulou-Soultani 2000; Andrade et al. 2011).
Our results provide evidence that temperature has a great influence on survival rates and developmental time of immature stages of V. canescens. Most parasitoids completed development successfully at medium temperatures (25 and 27.5°C), while at more extreme temperatures (17.5 and 32.5°C), survival was reduced significantly. According to Eliopoulos and Stathas (2003), using E. kuehniella as a host, the highest survival rates of V. canescens were recorded at 30 and 25°C (94.5 and 92.7%, respectively) whereas lower survival rates were observed at 15, 31, and 32°C (16.4, 17.5, and 15%, respectively). Similarly, Rahman et al. (2007) observed high survival rates for V. canescens when reared at temperatures ranging from 20 to 28°C (80–92%). Low survival rates of V. canescens at low temperatures can be explained by the fact that at such temperatures, host mortality risk is extremely high and furthermore, fitness of both host and parasitoid is affected significantly (Pennacchio and Strand 2006). On the other hand, higher temperatures increase host defense reactions (Fellowes et al. 1999). Moreover, immune components in the host plasma, such as prophenoloxidase (PPOs), become more active at temperatures above the optimal activation temperature (Zufelato et al. 2004). This possible scenario may explain the low survival rates of V. canescens that were recorded at high temperatures (30 and 32.5°C).
At 15°C, no individual completed development successfully. However, according to Eliopoulos and Stathas (2003), who used E. kuehniella as a host, a small proportion of V. canescens succeeded in completing development at 15°C (survival rate was estimated to 16.4%), which lasted 104.1 ± 3.2 days. Similarly, Rahman et al. (2007) observed that, when using E. kuehniella as a host, 40% of V. canescens succeeded in completing development at 15°C with mean developmental time estimated to 82.1 ± 1.3 days. This difference between our results and the previous ones is justified by the fact that P. interpunctella is considered to be less tolerant to low temperatures in comparison to E. kuehniella. Bell (1975) observed that larvae of P. interpunctella did not complete development successfully at 15°C, whereas those of E. kuehniella did so. Possibly, the failure of larvae of P. interpunctella to develop successfully at low temperatures affected the survival and development of V. canescens. This conclusion is consistent with the trend that development of immature parasitoids in their hosts is influenced by host species as well as by its size, age, physiological condition, and mortality risks (Harvey and Thompson 1995; Hu and Vinson 2000; Pennacchio and Strand 2006).
Total developmental time (from parasitism to adult eclosion) decreased significantly as testing temperature increased, within the range 17.5–27.5°C. The same trend was observed for the larval (from parasitism to pupation) and pupal (from pupation to adult eclosion) stage development time. According to Davidowitz and Nijhout (2004), metabolic rate of parasitoids increases as they are exposed to higher temperature regimes. The results of this work correspond with previous studies conducted using E. kuehniella as a host (Eliopoulos and Stathas 2003; Rahman et al. 2007).
In the current study, the lower developmental threshold of V. canescens, when using fifth instar larvae of P. interpunctella as a host, was estimated at 13.2°C. Previous studies using E. kuehniella as a host estimated the lower developmental threshold of V. canescens at 9.7°C (Rahman et al. 2007), 11.4°C (Ahmed 1936; Eliopoulos and Stathas 2003), and 13.3°C (Nakahara and Iwabuchi 2000). Differences are possible due to differences in host species, origin of the host and parasitoid population, host habitat, and number of temperatures tested (Campbell et al. 1974). The thermal constant was estimated at 312.5 DD, while previous studies calculated the same parameter at 307.4 and 404.3 DD (Eliopoulos and Stathas 2003; Rahman et al. 2007). Optimum temperature for total development and maximum temperature were calculated at 30.6 and 36.2°C, respectively. Similar values for optimal temperature and maximum temperature were calculated by Eliopoulos and Stathas (2003) (30.8 and 36.4°C, respectively).
The Logan I model described the effect of temperature on the developmental time of V. canescens well, as indicated by the values of the nonlinear regression coefficients (0.67–0.81), which were however slightly lower compared to the values of the linear model (0.82–0.89).
Adult longevity was significantly higher at lower temperature regimes (17.5–22.5°C) in relation to higher ones (27.5–32.5°C). This result is justified by the fact that low temperatures reduce metabolic rate of insects and as a result, adults survive for a longer period of time in relation to adults that live in habitats with higher temperatures (Davidowitz and Nijhout 2004). In the case of the parasitic wasp Meteorus trachynotus (Hymenoptera: Braconidae), female adults lived for a significantly longer period of time when reared at 15°C (40 days) compared to those reared at 30°C (10 days) (Thireau and Régnière 1995). The same trend was observed to adult individuals of the egg parasitoid Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae), which lived for a shorter period of time as temperature increased (Pizzol et al. 2010).
The data presented here provide fundamental information to understand the effect of temperature on some aspects of the biology of V. canescens, like survival and developmental time, and is of great importance for optimizing mass culturing of this parasitoid, so that it can be used as a biological agent in IPM programs against lepidopterous pests of stored products. According to our results, the optimal temperature for mass rearing, between those that were tested, was 25°C. Development at this temperature resulted in high survival, short developmental time, and relatively high adult longevity of V. canescens. However, even a slight increase of temperature by only 2.5°C led to a 2.2-fold decrease of adult longevity suggesting that temperature is a critical factor for the development of V. canescens. Parameters calculated in our experiment could be used for different populations of V. canescens reared on the same or different hosts. Further studies are required to determine whether V. canescens has the potential of becoming an effective biological control agent against P. interpunctella and other lepidopterous pests, under natural conditions.
References
Ahmed T (1936) The influence of ecological factors on the Mediterranean Flour Moth, Ephestia küehniella and its parasite Nemeritis canescens. J Anim Ecol 5:67–93
Andrade GS, Pratissoli D, Dalvi LP, Desneux N, Gonçalves dos Santos Junior HJ (2011) Performance of four Trichogramma species (Hymenoptera: Trichogrammatidae) as biocontrol agents of Heliothis virescens (Lepidoptera: Noctuidae) under various temperature regimes. J Pest Sci 84:313–320
Ashby MD, Singh P, Clare GK (1985) Cydia pomonella. In: Singh P, Moore RF (eds) Handbook of insect rearing, vol II. Elsevier Science Publishers, Amsterdam, pp 237–248
Begon M, Sait SM, Thompson DJ (1995) Persistence of a Parasitoid-host system: refuges and generation cycles? Proc R Soc Lond Biol Sci 260:131–137
Bell CH (1975) Effects of temperature and humidity on development of four pyralid moth pests of stored products. J Stored Prod Res 11:167–175
Bellows TS, Hassell MP (1988) The dynamics of age-structured host–parasitoid interactions. J Anim Ecol 57:259–268
Campbell A, Frazer BD, Gilbert N, Gutierrez AP, Mackauer M (1974) Temperature requirements of some aphids and their parasites. J Appl Ecol 11:431–438
Cossins AR, Bowler K (1987) Temperature biology of animals. Chapman and Hall, New York
Davidowitz G, Nijhout HF (2004) The physiological basis of reaction norms: the interaction among growth rate, the duration of growth and body size. Integr Comp Biol 44:443–449
Driessen G, Bernstein C (1999) Patch departure mechanisms and optimal host exploitation in an insect parasitoid. J Anim Ecol 68:445–459
Eliopoulos PA, Stathas GJ (2003) Temperature-dependent development of the koinobiont endoparasitoid Venturia canescens (Hymenoptera: Ichneumonidae): effect of host instar. Environ Entomol 32:1049–1055
Eliopoulos PA, Harvey JA, Athanassiou CG, Stathas GJ (2003) Effect of biotic and abiotic factors on reproductive parameters of the synovigenic endoparasitoid Venturia canescens. Physiol Entomol 28:268–275
Fellowes MDE, Kraaijeveld AR, Godfray HCJ (1999) Cross-resistance following artificial selection for increased defense against parasitoids in Drosophila melanogaster. Evolution 53:966–972
Got B, Piry S, Migeon A, Labatte LM (1997) Comparison of different models for predicting development time of the European corn borer (Lepidoptera: Pyralidae). Environ Entomol 26:46–60
Hallmann GJ, Denlinger DL (1998) Introduction: temperature sensitivity and integrated pest management. In: Hallman GJ, Denlinger DL (eds) Temperature sensitivity in insects and application in integrated pest management. Westview Press, Boulder, pp 1–5
Harvey JA, Thompson DJ (1995) Developmental interactions between the solitary endoparasitoid Venturia canescens (Hymenoptera: Ichneumonidae) and two of its hosts, Plodia interpunctella and Corcyra cephalonica (Lepidoptera: Pyralidae). Eur J Entomol 92:427–435
Harvey JA, Harvey IF, Thompson DJ (1993) The effect of superparasitism on development of the solitary parasitoid wasp Venturia canescens (Hymenoptera: Ichneumonidae). Ecol Entomol 18:203–208
Harvey JA, Harvey IF, Thompson DJ (2001) Lifetime reproductive success in the solitary endoparasitoid, Venturia canescens. J Insect Behav 14:573–593
Higley LG, Pedigo LP, Ostile KR (1986) DEGDAY: a program for calculating degree days, and assumptions behind the degree day approach. Environ Entomol 15:999–1016
Hu JS, Vinson SB (2000) Interaction between the larval endoparasitoid Campoletis sonorensis (Hymenoptera: Ichneumonidae) and its host the tobacco budworm (Lepidoptera: Noctuidae). Ann Entomol Soc Am 93:220–224
Hubbard SF, Cook RM (1978) Optimal foraging by parasitoid wasps. J Anim Ecol 47:593–604
Institute SAS (1989) JMP, A guide to statistical and data analysis, v.4.02. SAS Institute Inc., Cary
Logan JA, Hoyt DJ, Tanigoshi LK (1976) An analytic model for description of temperature dependent rate phenomena in arthropods. Environ Entomol 5:1133–1140
Lucchetta P, Bernstein C, Théry M, Lazzari C, Desouhant E (2008) Foraging and associative learning of visual signals in a parasitic wasp. Anim Cogn 11:525–533
Milonas PG, Savopoulou-Soultani M (2000) Temperature dependent development of the parasitoid Colpoclypeus florus (Hymenoptera: Eulophidae) in the laboratory. J Econ Entomol 93:1627–1632
Nakahara Y, Iwabuchi K (2000) Investigation of low thermal threshold for development of the larval endoparasitoid, Venturia canescens (Hymenoptera: Ichneumonidae) using in vitro culture technique. Entomol Sci 3:19–23
Ozkan C (2007) Effect of food, light and host instar on the egg load of the synovigenic endoparasitoid Venturia canescens (Hymenoptera: Ichneumonidae). J Pest Sci 80:79–83
Pennacchio F, Strand MR (2006) Evolution of developmental strategies in parasitic Hymenoptera. Ann Rev Entomol 51:233–258
Pizzol J, Pintureau B, Khoualdia O, Desneux N (2010) Temperature-dependent differences in biological traits between two strains of Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae). J Pest Sci 83:447–452
Rahman MM, Roberts HLS, Schmidt O (2007) Factors affecting growth in the koinobiont endoparasitoid Venturia canescens in the flour moth Ephestia kuehniella. J Insect Physiol 53:463–467
Rogers D (1972) The ichneumon wasp Venturia canescens: Oviposition and avoidance of superparasitism. Entomol Exp Appl 15:190–194
Salt G (1975) The fate of an internal parasitoid, Nemeritis canescens, in a variety of insects. Trans R Ent Soc London 127:141–461
Schneider MV, Beukeboom LW, Driessen G, Lapchin L, Bernstein C, van Alphen JJM (2002) Geographical distribution and genetic relatedness of sympatrical thelytokous and arrhenotokous populations of the parasitoid Venturia canescens (Hymenoptera). J Evol Biol 15:191–200
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. Freeman, New York
Speight MR, Hunter MD, Watt AD (1999) Ecology of insects: concepts and applications. Blackwell Scientific Publications, London
SPSS Inc. (2006) SPSS for Windows, Version 15.0, Chicago
Thiel A, Driessen G, Hoffmeister TS (2006) Different habitats, different habits? Response to foraging information in the parasitic wasp Venturia canescens. Behav Ecol Sociobiol 59:614–623
Thireau JC, Régnière J (1995) Development, reproduction, voltinism and host synchrony of Meteorus trachynotus with its hosts Choristoneura fumiferana and C. rosaceana. Entomol Exp Appl 76:67–82
Zufelato MS, Lourenco AP, Simoes ZLP, Jorge JA, Bitondi MMG (2004) Phenoloxidase activity in Apis mellifera honey bee pupae, and ecdysteroid-dependent expression of the prophenoloxidase mRNA. Insect Biochem Mol 34:1257–1268
Acknowledgment
The authors express their thanks to Mrs Christina Soultani for her constructive comments on an early version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Zalucki.
Rights and permissions
About this article
Cite this article
Spanoudis, C.G., Andreadis, S.S. Temperature-dependent survival, development, and adult longevity of the koinobiont endoparasitoid Venturia canescens (Hymenoptera: Ichneumonidae) parasitizing Plodia interpunctella (Lepidoptera: Pyralidae). J Pest Sci 85, 75–80 (2012). https://doi.org/10.1007/s10340-011-0405-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-011-0405-y