Abstract
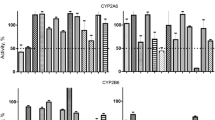
F18, N-hydroxy-4-(2-methoxy-5-(methyl (2-methylquinazolin-4-yl) amino) phenoxy) butanamide, is a novel selective HDAC6 inhibitor with good antitumor activity. In the early drug development, drug-metabolism studies are a crucial and indispensable part. In this study, we proposed to evaluate the in vitro primary metabolism of F18 in phase Ι in liver microsomes from human, rat, dog, monkey and mouse and investigate the metabolite profile both in vitro and in vivo using LC–MS/MS methods. F18 showed high metabolic stability in human, rat, dog, monkey and mouse liver microsomes over 120 min, with t 1/2 >8 h in human, rat, and dog, and t 1/2 <3.5 h in monkey, with almost no clearance in mouse. Human cytochrome P450 (P450) phenotyping showed that F18 was predominantly metabolized by CYP2C9, CYP2E1, CYP2D6 and CYP3A4. The investigation of the effect of F18 on CYP enzymes in HLM demonstrated that this compound did not significantly inhibit CYP 1A2 (IC50 >100 μM), was a moderate inhibitor of CYP3A4 (IC50 = 1.63 μM) and had negligible effects on CYP3A1/2 activity in rats. The results will be valuable in understanding drug–drug interactions (DDI) when F18 is co-administered with other drugs. The metabolites of F18 were investigated in rat plasma, urine, feces and different liver microsomes in NADPH samples, yielding at least 11 metabolites in these biological samples. The prominent metabolic pathways were de-methylation, de-amination, de-oxidation and O-glucuronidation. In summary, this work provides the first clues regarding F18 metabolism, providing important information for comprehensive understanding of F18 metabolites.





Similar content being viewed by others
References
Kim HM, Oh SJ, Park SK, Han G, Kim K, Lee KS, Kang JS, Nan M, Lee K (2008) In vitro metabolism of KBH-A40, a novel δ-lactam-based histone deacetylase (HDAC) inhibitor, in human liver microsomes and serum. Xenobiotica 38:281–293
Wang HS, Yu NF, Chen DZ, Lee KCL, Lye PL, Chang JWW, Deng WP, Ng MCY, Lu T, Khoo ML, Poulsen A, Sangthongpitag K, Wu XF, Hu CY, Goh KC, Wang XK, Fang LJ, Goh KL, Khng HH, Goh SK, Yeo P, Liu X, Bonday Z, Wood JM, Dymock BW, Ethirajulu K, Sun ET (2011) Discovery of (2E)-3-{2-butyl-1-[2-(diethylamino)ethyl]-1H benzimidazol-5-yl}-N-hydroxyacrylamide (SB939), an orally active histone deacetylase inhibitor with a superior preclinical profile. J Med Chem 54:4694–4720
Michan S, Sinclair D (2007) Sirtuins in mammals: insights into their biological function. Biochem J 404:1–13
Otaegui D, Gascón AR, Zubia A, Cossío FP, Pedraz JL (2009) Pharmacokinetics and tissue distribution of Kendine 91, a novel histone deacetylase inhibitor, in mice. Cancer Chemother Pharm 64:153–159
Duvic M, Talpur R, Ni X, Zhang C, Hazarika P, Kelly C, Chiao JH, Reilly JF, Ricker JL, Richon VM, Frankel SR (2007) Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL). Blood 109:31–39
Atadja P (2009) Development of the pan-DAC inhibitor panobinostat (LBH589): successes and challenges. Cancer Lett 280(2):233–241
Ramalingam SS, Belani CP, Ruel C, Frankel P, Gitlitz B, Koczywas M, Delgado LE, Gandara D (2009) Phase II study of belinostat (PXD101), a histone deacetylase inhibitor, for second line therapy of advanced malignant pleural mesothelioma. J Thorac Oncol 4:97–101
Gryder BE, Sodji QH, Oyelere AK (2012) Targeted cancer therapy: giving histone deacetylase inhibitors all they need to succeed. Future Med Chem 4:505–524
Boumber Y, Younes A, Garcia-Manero G (2011) Mocetinostat (MGCD0103): a review of an isotype-specific histone deacetylase inhibitor. Expert Opin Invest Drugs 20:823–829
Wang HS, Dymock WB (2009) New patented histone deacetylase inhibitors. Expert Opin Ther Patens 19:1727–1757
Kovacs JJ, Murphy PJM, Gaillard S, Zhao X, Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, Yao TP (2005) HDAC 6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell 18:601–607
Zhang Y, Li N, Caron C, Matthias G, Hess D, Khochbin S, Matthias P (2003) HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J 22(5):1168–1179
Santo L, Hideshima T, Kung AL, Tseng JC, Tamang D, Yang M, Jarpe M, Duzer JHV, Mazitschek R, Ogier WC, Cirstea D, Rodig S, Eda H, Scullen T, Canavese M, Bradner J, Anderson KC, Jones SS, Raje N (2012) Preclinical activity, pharmacodynamic, and pharmacokinetic properties of a selective HDAC6 inhibitor, ACY-1215, in combination with bortezomib in multiple myeloma. Blood 119:2579–2589
Yang Z, Wang TJ, Wang F, Niu T, Liu ZW, Chen XX, Long CF, Tang MH, Cao D, Wang XY, Xiang W, Yi YY, Ma L, You JS, Chen LJ (2016) Discovery of selective histone deacetylase 6 inhibitors using the quinazolin as the cap for the treatment of cancer. J Med Chem 59(4):1455–1470
Huang JG, Si LQ, Fan ZZ, Hu L, Qiu J, Li G (2011) In vitro metabolic stability and metabolite profiling of TJ0711 hydrochloride, a newly developed vasodilatory β-blocker, using a liquid chromatography-tandem mass spectrometry method. J Chromatogr B 879:3386–3392
Messianoa GB, Santosb RAS, Ferreirac LS, Simões RA, Jaborc VAP, Katod MJ, Lopesc NP, Pupoa MT, Oliveira ARM (2013) In vitro metabolism study of the promising anticancer agent the lignan (−)-grandisin. J Pharmaceut Biomed 72:240–244
Iwatsubo T, Hirota N, Ooie T, Suzuki H, Shimada N, Chiba K (1997) Prediction of in vivo drug metabolism in the human liver from in vitro metabolism data. Pharmacol Ther 73:147–171
Ghanbari F, Rowland YK, Bloomer JC, Clarke SE, Lennard MS, Tucker GT, Rostami-Hodjegan A (2006) A critical evaluation of the experimental design of studies of mechanism based enzyme inhibition, with implications for in vitro–in vivo extrapolation. Drug Metab Rev 7(3):315–334
Gombar VK, Silver IS, Zhao Z (2003) Role of ADME Characteristics in drug discovery and their in silico evaluation: in silico screening of chemicals for their metabolic stability. Curr Top Med Chem 3:1205–1225
Guo W, Shi XW, Wang W, Zhang WL, Li JX (2014) Identification of the rat liver cytochrome P450 enzymes involved in the metabolism of the calcium channel blocker dipfluzine hydrochloride. Environ Toxicol Pharm 38:901–902
Wang CY, Dong YF, Zhao H, Wang CY, Li R, Qiu N, Ye HY, Tang MH, Chen LJ (2015) Characterization of in vitro primary metabolic profile of SKLB-M8, a novel antitumor compound, using liquid chromatography coupled with triple quadrupole tandem mass spectrometry and quadrupole time-of-flight tandem mass spectrometry. Int J Mass Spectrom 383–384:23–30
Hickman D, Wang JP, Wang Y, Unadkat JD (1998) Evaluation of the selectivity of in vitro probes and suitability of organic solvents for the measurement of human cytochrome P450 monooxygenase activities. Drug Metab Dispos 26:207–215
Yuan R, Madani S, Wei XX, Reynolds K, Huang SM (2002) Evaluation of cytochrome P450 probe substrates commonly used by the pharmaceutical industry to study in vitro drug interactions. Drug Metab Dispos 30:1311–1319
Videau O, Pitarque S, Troncale S, Hery P, Thevenot E, Delaforge M, Benech H (2012) Can a cocktail designed for phenotyping pharmacokinetics and metabolism enzymes in human be used efficiently in rat? Xenobiotica 42:349–354
Baranczewski P, Stańczak A, Sundberg K, Svensson R, Wallin Å, Jansson J, Garberg P, Postlind H (2006) Introduction to in vitro estimation of metabolic stability and drug interactions of new chemical entities in drug discovery and development. Pharmacol Rep 58:453–472
Houston JB (1994) Utility of in vitro drug metabolism data in predicting in vivo metabolic clearance. Biochem Pharmacol 47:1469–1479
Obach RS, Baxter JG, Liston TE, Silber BM, Jones BC, Macintyre F, Rance DJ, Wastall P (1997) The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J Pharmacol Exp Ther 283:46–58
Li X, Wang K, Wei W, Liu YY, Gong L (2013) In vitro metabolism of brucine by human liver microsomes and its interactions with CYP substrates. Chem Biol Interact 204:140–143
Scripture CD, Figg WD (2006) Drug interactions in cancer therapy. Nat Rev Cancer 6:546–558
Yan Z, Caldwell GW (2001) Metabolism profiling, and cytochrome P450 inhibition & induction in drug discovery. Curr Top Med Chem 1:403–425
Liu L, Xiao J, Peng ZH, Wu WW, Du P, Chen Y (2012) In vitro metabolism of strychnine by human cytochrome P450 and its interaction with glycyrrhetic acid. Chin Herb Med 4:118–125
Du L, Musson DG, Wang AQ (2005) High turbulence liquid chromatography online extraction and tandem mass spectrometry for the simultaneous determination of suberoylanilide hydroxamic acid and its two metabolites in human serum. Rapid Commun Mass Spectrom 19:1779–1787
Dong YF, Tang MH, Song H, Li R, Wang CY, Ye HY, Qiu N, Zhang YK, Chen LJ, Wei YQ (2014) Characterization of metabolic profile of honokiol in rat feces using liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry and 13C stable isotope labeling. J Chromatogr B 953–954:20–29
Acknowledgments
The authors state no conflicts of interest. This work was supported by the National Key Programs of China during the 12th Five-Year Plan Period (2012ZX09103101-009) and Guangdong Innovative Research Team Program (no. 2011Y073).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Whole procedures involving animals had been approved by the Institute Guidelines on Animal Experimentation of Sichuan University in China.
Additional information
X. Li and M. Tang are joint first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, X., Tang, M., Wang, H. et al. In Vitro and In Vivo Primary Metabolic Characterization of F18, a Novel Histone Deacetylase-6 (HDAC6) Inhibitor, Using UHPLC–QqQ–MS/MS and Q-TOF–MS Methods. Chromatographia 79, 1479–1490 (2016). https://doi.org/10.1007/s10337-016-3163-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-016-3163-4