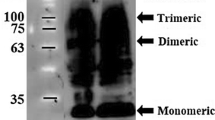
Abstract
Affinity chromatography strategies using amino acids as immobilize d ligands have been successfully applied for the purification of different biomolecules from complex mixtures. Therefore, in this work, several supports with immobilized amino acids were applied for the purification of membrane-bound catechol-O-methyltransferase (MBCOMT) from Pichia pastoris lysates and it was verified that l-arginine provided the required selectivity for MBCOMT isolation. The optimization of the binding and elution buffers composition allowed the recovery of purified MBCOMT in a biological and immunologically active state from the arginine support. Additional optimization experiments varying the mobile phase pH, temperature and the concentration of the injected sample were carried out and an improvement of MBCOMT adsorption and purity was observed. Indeed, the optimized conditions for MBCOMT isolation and purification consisted in: loading of 4 mg of total protein onto the column previously equilibrated at 20 °C where the target enzyme was recovered in a purified fraction using 500 mM NaCl, 10 mM DTT and 0.5 % (v/v) Triton X-100 in 10 mM Tris buffer (pH 7) with a total bioactivity recovery of 24 ± 2.2 % and a purification fold of 4.95 ± 0.23, a value that is consistent with the best values ever reported for MBCOMT. Moreover, the l-arginine support demonstrated the ability to bind the target protein in a wide range of pH values (above and below the pI of the target protein) and the MBCOMT elution occurs in a single peak pattern. Finally, the strategy here reported can aid in the implementation of crystallization studies with MBCOMT in complex with clinically relevant inhibitors since it is obtained in a purified form with biological activity. In conclusion, a novel affinity chromatography strategy was developed and implemented for recombinant MBCOMT purification in a highly immunological and biologically active state.


Similar content being viewed by others
Abbreviations
- BMGH:
-
Buffered minimal glycerol
- BMMH:
-
Buffered minimal methanol
- COMT:
-
Catechol-O-methyltransferase
- DTT:
-
Dithiothreitol
- MBCOMT:
-
Membrane-bound catechol-O-methyltransferase
- P. pastoris :
-
Pichia pastoris
- YNB:
-
Yeast nitrogen base
- YPD:
-
Yeast extract peptone dextrose
References
Bonifacio MJ, Palma PN, Almeida L, Soares-da-Silva P (2007) Catechol-O-methyltransferase and its inhibitors in Parkinson’s disease. CNS Drug Rev 13:352–379
Karege F, Bovier P, Gaillard JM, Tissot R (1987) The decrease of erythrocyte catechol-O-methyltransferase activity in depressed patients and its diagnostic significance. Acta Psychiatr Scand 76:303–308
Yager JD, Liehr JG (1996) Molecular mechanisms of estrogen carcinogenesis. Annu Rev Pharmacol Toxicol 36:203–232
Pedro AQ, Bonifácio MJ, Queiroz JA, Maia CJ, Passarinha LA (2011) A novel prokaryotic expression system for biosynthesis of recombinant human membrane-bound catechol-O-methyltransferase. J Biotechnol 156:141–146
Correia FF, Santos FM, Pedro AQ, Bonifácio MJ, Queiroz JA, Passarinha LA (2014) Recovery of biological active catechol-O-methyltransferase isoforms from Q-sepharose. J Sep Sci 37:20–29
Santos FM, Pedro AQ, Soares RF, Martins R, Bonifácio MJ, Queiroz JA, Passarinha LA (2013) Performance of hydrophobic interaction ligands for human membrane-bound catechol-O-methyltransferase purification. J Sep Sci 36:1693–1702
Smith SM (2011) Strategies for the purification of membrane proteins. Methods Mol Biol 681:485–496
Seddon AM, Curnow P, Booth PJ (2004) Membrane proteins, lipids and detergents: not just a soap opera. Biochim Biophys Acta 1666:105–117
Zou H, Luo Q, Zhou D (2011) Affinity membrane chromatography for the analysis and purification of proteins. J Biochem Biophys Methods 49:199–240
Costa SR, Bonifácio MJ, Queiroz JA, Passarinha LA (2011) Analysis of hSCOMT adsorption in bioaffinity chromatography with immobilized amino acids: the influence of pH and ionic strength. J Chromatogr B Analyt Technol Biomed Life Sci 879:1704–1706
Martins R, Queiroz JA, Sousa F (2013) New approach in RNA quantification using arginine-affinity chromatography: potential application in eukaryotic and chemically synthesized RNA. Anal Bioanal Chem 405:8849–8858
Sousa F, Prazeres DMF, Queiroz JA (2009) Improvement of transfection efficiency by using supercoiled plasmid DNA purified with arginine affinity chromatography. J Gene Med 11:79–88
Sousa F, Prazeres DMF, Queiroz JA (2008) Affinity chromatography approaches to overcome the challenges of purifying plasmid DNA. Trends Biotechnol 26:518–525
Sousa A, Sousa F, Queiroz JA (2009) Selectivity of arginine chromatography in promoting different interactions using synthetic oligonucleotides as model. J Sep Sci 32:1665–1672
Vijayalakshmi MA (1996) Histidine ligand affinity chromatography. Mol Biotechnol 6:347–357
Pereira P, Sousa A, Queiroz J, Correia I, Figueiras A, Sousa F (2014) Purification of pre-miR-29 by arginine-affinity chromatography. J Chromatogr B Analyt Technol Biomed Life Sci 951:16–23
Patel A, O’Hara M, Callaway JE, Green D, Martin J, Nishikawa AH (1990) Affinity purification of tissue plasminogen activator using transition-state analogues. J Chromatogr 510:83–93
Suzuki T, Takahashi H (1974) Purification of prekallikrein with arginine-agarose. Methods Enzymol 34:432–435
Sakuragawa N, Takahashi K, Ashizawa T (1977) Isolation of prothrombin. Acta Med Biol 25:119–125
Pedro AQ, Oppolzer D, Bonifácio MJ, Maia CJ, Queiroz JA, Passarinha LA (2015) Evaluation of MutS and Mut+ Pichia pastoris strains for membrane-bound and catechol-O-methyltransferase biosynthesis. Appl Biochem Biotechnol 175:3840–3855
Vieira-Coelho MA, Soares-da-Silva P (1999) Effects of tolcapone upon soluble and membrane-bound brain and liver catechol-O-methyltransferase. Brain Res 821(1):69–78
Pedro AQ, Soares RF, Oppolzer D, Santos FM, Rocha LA, Gonçalves AM, Bonifácio MJ, Queiroz JA, Gallardo E, Passarinha LA (2014) An improved HPLC method for quantification of metanephrine with coulometric detection. J Chromatograph Sep Tech 5:217
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Passarinha LA, Bonifacio MJ, Queiroz JA (2007) Comparative study on the interaction of recombinant human soluble catechol-O-methyltransferase on some hydrophobic adsorbents. Biomed Chromatogr 21:430–438
Cotton NJ, Stoddard B, Parson WW (2004) Oxidative inhibition of human soluble catechol-O-methyltransferase. J Biol Chem 279(22):23710–23718
Lee D, Lee J, Seok C (2013) What stabilizes close arginine pairing in proteins? Phys Chem Chem Phys 15:5844–5853
Queiroz JA, Tomaz CT, Cabral JMS (2001) Hydrophobic interaction chromatography of proteins. J Biotechnol 87:143–159
Ahuja S (2003) Chromatography and separation science. Academic Press, San Diego
Bonifacio MJ, Archer M, Rodrigues ML, Matias PM, Learmonth DA, Carrondo MA, Soares-Da-Silva (2002) Kinetics and crystal structure of catechol-O-methyltransferase complex with co-substrate and a novel inhibitor with potential therapeutic application. Mol Pharmacol 62(4):795–805
Rodrigues ML, Bonifacio MJ, Soares-Da-Silva P, Carrondo MA, Archer M (2005) Crystallization and preliminar X-ray diffraction studies if a catechol-O-methyltransferase/inhibitor complex. Acta Crystallogr Sect F Struct Biol Cryst Commun 61(1):118–120
Acknowledgments
This research was supported by University of Beira Interior—Health Sciences Research Centre (CICS) and FCT (Portuguese Foundation for Sciences and Technology) by the project “EXPL/BBB478/BQB/0960/2012” and COMPETE: FCOMP-01-0124-FEDER-027563. A. Q. Pedro and P. Pereira acknowledge a doctoral fellowship (SFRH/BD/81222/2011 and SFRH/BD/81914/2011) from Fundação para a Ciência e Tecnologia. The authors also acknowledge the program COMPETE, the FCT project (Pest-C/SAU/UI0709/2011).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no commercial or financial conflict of interests. In this work, no research involving human participants or animals was carried out.
Rights and permissions
About this article
Cite this article
Pedro, A.Q., Pereira, P., Bonifácio, M.J. et al. Purification of Membrane-Bound Catechol-O-Methyltransferase by Arginine-Affinity Chromatography. Chromatographia 78, 1339–1348 (2015). https://doi.org/10.1007/s10337-015-2970-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-015-2970-3