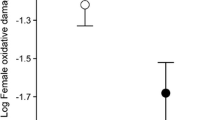
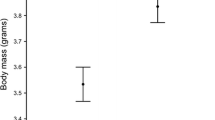
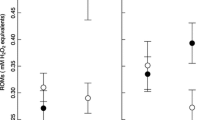
Abstract
Avian mothers can influence the fitness of their offspring by resource investment into the egg. Allocation of macro- and micronutrients into the eggs may be costly for the female, therefore, we expect that resource investment may be affected by the environmental and social conditions the mother experiences during egg formation. Here, we investigated whether environmental circumstances experienced by the reproducing female exert an influence on egg mass, yolk antioxidant (lutein and tocopherol) concentration, eggshell thickness, and the eggshell spotting patterns of Great Tits (Parus major). Our study showed that when caterpillars were less abundant, in colder weather and when breeding in an area of higher local breeding density, female Great Tits laid eggs of lower mass, suggesting that adverse environmental circumstances constrain the macronutrient investment into the eggs. However, we found no evidence that yolk antioxidant concentration and eggshell thickness were affected by environmental factors. Female Great Tits may use their endogenous stores or spend more time and energy in finding sufficient amounts of dietary antioxidants and calcium under unfavourable environmental conditions, which may have a cost effect on their own conditions. We found that birds that bred in the beginning of the season laid eggs with darker eggshell spots. Moreover, females laid more spotted eggs in colder weather and when breeding in higher density areas. Our results suggest that Great Tits deposit more of the potentially harmful pro-oxidant protoporphyrin pigment into the eggshell under unfavourable environmental conditions.
Zusammenfassung
Auswirkungen von Umweltbedingungen auf Eimasse, Antioxidantiengehalt des Dotters, Schalendicke und Fleckung der Eischale bei Kohlmeisen (Parus major)
Vogelmütter können die Fitness ihrer Nachkommenschaft durch die Ressourcen beeinflussen, die sie in ein Ei investieren. Die Abgabe von Makro- und Mikronährstoffen an die Eier kann für das Weibchen kostspielig sein, daher ist zu erwarten, dass die Investition von Ressourcen durch Umweltbedingungen und soziale Faktoren beeinflusst wird, denen die Mutter während der Eibildung ausgesetzt ist. Hier untersuchten wir, inwieweit die Umweltbedingungen, denen das Weibchen während der Fortpflanzung ausgesetzt war, einen Einfluss auf die Eimasse, die Konzentration von Antioxidantien (Lutein und Tocopherol) im Dotter, die Schalendicke und die Fleckung der Eischalen von Kohlmeisen (Parus major) ausübten. Unsere Studie zeigte, dass Kohlmeisen-Weibchen in Zeiten geringeren Raupenangebots, bei kälterem Wetter und in Brutgebieten mit höherer lokaler Brutdichte Eier von geringerer Masse legten, was darauf hindeutet, dass ungünstige Umweltbedingungen die Investition von Makronährstoffen in die Eier beschränken können. Allerdings fanden wir keine Hinweise darauf, dass die Konzentration von Antioxidantien im Dotter und die Schalendicke durch Umweltfaktoren beeinflusst wurden. Unter ungünstigen Umweltbedingungen können Kohlmeisen-Weibchen körpereigene Reserven angreifen oder mehr Zeit und Energie auf die Suche nach ausreichenden Mengen nahrungsgebundener Antioxidantien und Kalzium aufwenden, was zu Lasten ihrer eigenen Kondition gehen kann. Wir stellten fest, dass früh in der Saison brütende Vögel Eier mit dunklerer Schalenfleckung legten. Außerdem legten die Weibchen bei kälterem Wetter und in dichter besetzten Brutgebieten mehr gefleckte Eier. Unsere Ergebnisse deuten an, dass Kohlmeisen unter ungünstigen Umweltbedingungen mehr des potenziell schädlichen prooxidativen Pigments Protoporphyrin in der Eischale ablagern.





Similar content being viewed by others
References
Afonso S, Vanore G, Batlle A (1999) Protoporphyrin IX and oxidative stress. Free Radic Res 31:161–170
Alatalo RV, Lundberg A (1984) Density-dependence in breeding success of the pied flycatcher (Ficedula hypoleuca). J Anim Ecol 53:969–977
Ardia DR, Wasson MF, Winkler DW (2006) Individual quality and food availability determine yolk and egg mass and egg composition in tree swallows Tachycineta bicolor. J Avian Biol 37:252–259
Arnold KE, Ramsay SL, Henderson L, Larcombe SD (2010) Seasonal variation in diet quality: antioxidants, invertebrates and blue tits Cyanistes caeruleus. Biol J Linn Soc 99:708–717
Avilés JM, Stokke BG, Moksnes A, Roskaft E, Møller AP (2007) Environmental conditions influence egg color of reed warblers Acrocephalus scirpaceus and their parasite, the common cuckoo Cuculus canorus. Behav Ecol Sociobiol 61:475–485
Blomqvist D, Johansson OC, Götmark F (1997) Parental quality and egg size affect chick survival in a precocial bird, the Lapwing Vanellus vanellus. Oecologia 110:18–24
Blount JD, Surai PF, Houston DC, Møller AP (2002) Patterns of yolk enrichment with dietary carotenoids in gulls: the roles of pigment acquisition and utilization. Funct Ecol 16:445–453
Both C (1998) Experimental evidence for density dependence of reproduction in Great Tits. J Anim Ecol 67:667–674
Brulez K, Cassey P, Meeson A, Mikšík I, Webber SL, Gosler AG, Reynolds SJ (2014) Eggshell spot scoring methods cannot be used as a reliable proxy to determine pigment quantity. J Avian Biol 45:94–102
Bryant DM (1975) Breeding biology of House Martins Delichon urbica in relation to aerial insect abundance. Ibis 117:180–216
Buchanan KL, Evans MR, Goldsmith AR, Bryant DM, Rowe LV (2001) Testosterone influences basal metabolic rate in male house sparrows: a new cost of dominance signalling? Proc R Soc B 268:1337–1344
Bureš S, Weidinger K (2003) Sources and timing of calcium intake during reproduction in flycatchers. Oecologia 137:634–647
Christians JK (2002) Avian egg size: variation within species and inflexibility within individuals. Biol Rev 77:1–26
Clotfelter ED, O’Neal DM, Gaudioso JM, Casto JM, Parker-Renga IM, Snajdr EA, Duffy DL, Nolan V Jr, Ketterson ED (2004) Consequences of elevating plasma testosterone in females of a socially monogamous songbird: evidence of constraints on male evolution? Horm Behav 46:171–178
Cucco M, Guasco B, Ottonelli R, Balbo V, Malacarne G (2009) The influence of temperature on egg composition in the grey partridge Perdix perdix. Ethol Ecol Evol 21:63–77
Duval C, Cassey P, Mikšík I, Reynolds SJ, Spencer KA (2013) Condition-dependent strategies of eggshell pigmentation: an experimental study of Japanese quail (Coturnix coturnix japonica). J Exp Biol 216:700–708
Eeva T, Lehikoinen E (1995) Egg shell quality, clutch size and hatching success of the Great Tit (Parus major) and the pied flycatcher (Ficedula hyoleuca) in an air pollution gradient. Oecologia 102:312–323
Eeva T, Helle S, Salminen JP, Hakkarainen H (2010) Carotenoid composition of invertebrates consumed by two insectivorous bird species. J Chem Ecol 36:608–613
Encabo SI, Monrós JS, Barba E (2001) Egg size variation in a Mediterranean Great Tit Parus major population. Ardeola 48:63–70
Garamszegi LZ, Török J, Tóth L, Michl G (2004) The effect of timing and female quality on clutch size in the Collared Flycatcher Ficedula albicollis. Bird Study 51:270–277
García-Navas V, Sanz JJ, Merino S, Martínez-de la Puente J, Lobato E, del Cerro S, Rivero J, Ruiz de Castañeda R, Moreno J (2010) Experimental evidence for the role of calcium in eggshell pigmentation pattern and breeding performance in Blue Tits Cyanistes caeruleus. J Ornithol 152:71–82
Goodwin TW (1984) The biochemistry of the carotenoids. II. Animals. Chapman & Hall, London
Gorchein A, Lim CK, Cassey P (2009) Extraction and analysis of colourful eggshell pigments using HPLC and HPLC/electrospray ionization tandem mass spectrometry. Biomed Chromatogr 23:602–606
Gosler AG, Barnett PR, Reynolds SJ (2000) Inheritance and variation in eggshell patterning in the Great Tit Parus major. Proc R Soc Lond B 267:2469–2473
Gosler AG, Higham JP, Reynolds SJ (2005) Why are birds’ eggs speckled? Ecol Lett 8:1105–1113
Graveland J (1996) Avian eggshell formation in calcium-rich and calcium-poor habitats: importance of snail shells and anthropogenic calcium sources. Can J Zool 74:1035–1044
Graveland J, Berends JE (1997) Timing of the calcium intake and effect of calcium deficiency on behaviour and egg laying in captive Great tits Parus major. Physiol Zool 70:74–84
Graveland J, van Gijzen T (1994) Arthropods and seeds are not sufficient as calcium sources for shell formation and skeletal growth in passerines. Ardea 82:299–314
Graveland J, van der Wal R, van Balen JH, van Noordwijk AJ (1994) Poor reproduction in forest passerines from decline of snail abundance in acidified soils. Nature 368:446–448
Haftorn S (1985) Variations in clutch size and egg dimensions of the great tit Parus major. Fauna Norvegica C 8:106–115
Halliwell B, Gutteridge JMC (2007) Free radicals in biology and medicine. Oxford University Press, Oxford
Hargitai R, Török J, Tóth L, Hegyi G, Rosivall B, Szigeti B, Szöllősi E (2005) Effects of environmental conditions and parental quality on inter-and intraclutch egg-size variation in the Collared Flycatcher (Ficedula albicollis). Auk 122:509–522
Hargitai R, Matus Z, Hegyi G, Michl G, Tóth G, Török J (2006) Antioxidants in the egg yolk of a wild passerine: differences between breeding seasons. Comp Biochem Physiol B 143:145–152
Hargitai R, Herényi M, Török J (2008) Eggshell colouration in relation to female condition, male attractiveness and egg quality in the collared flycatcher (Ficedula albicollis). J Avian Biol 39:413–422
Hargitai R, Arnold KE, Herényi M, Prechl J, Török J (2009) Egg composition in relation to social environment and maternal physiological condition in the collared flycatcher. Behav Ecol Sociobiol 63:869–882
Hargitai R, Moskát C, Bán M, Gil D, López-Rull I, Solymos E (2010) Eggshell characteristics and yolk composition in the common cuckoo Cuculus canorus: are they adapted to brood parasitism? J Avian Biol 41:177–185
Hargitai R, Mateo R, Török J (2011) Shell thickness and pore density in relation to shell colouration, female characteristics, and environmental factors in the Collared Flycatcher Ficedula albicollis. J Ornithol 152:579–588
Hargitai R, Nagy G, Herényi M, Török J (2013) Effects of experimental calcium availability, egg parameters, and laying order on Great Tit Parus major eggshell pigmentation patterns. Ibis 155:561–570
Hargitai R, Nagy G, Nyiri Z, Bervoets L, Eke Zs, Eens M, Török J (2016a) Effects of breeding habitat (woodland versus urban) and metal pollution on the egg characteristics of great tits (Parus major). Sci Total Environ 544:31–38
Hargitai R, Nagy G, Herényi M, Nyiri Z, Laczi M, Hegyi G, Eke Zs, Török J (2016b) Darker eggshell spotting indicates lower yolk antioxidant level and poorer female quality in the Great Tit (Parus major). The Auk 133:131–146
Hargitai R, Nyiri Z, Eke Zs, Török J (2016c) Effects of temperature and duration of storage on the stability of antioxidant compounds in egg yolk and plasma. Physiol Biochem Zool 89:161–167
Honza M, Procházka P, Požgayová M (2012) Do weather conditions affect the colouration of great reed warbler Acrocephalus arundinaceus eggs? Folia Zool 61:219–224
Hõrak P, Surai PF, Møller AP (2002) Fat-soluble antioxidants in the eggs of Great Tits Parus major in relation to breeding habitat and laying sequence. Avian Sci 2:123–130
Isaksson C, Andersson S (2007) Carotenoid diet and nestling provisioning in urban and rural great tits Parus major. J Avian Biol 38:564–572
Isaksson C, Johansson A, Andersson S (2008) Egg yolk carotenoids in relation to habitat and reproductive investment in the Great Tit Parus major. Physiol Biochem Zool 81:112–118
Järvinen A (1991) Proximate factors affecting egg volume in subarctic hole-nesting passerines. Ornis Fennica 68:99–104
Kennedy GY, Vevers HG (1976) A survey of avian eggshell pigments. Comp Biochem Physiol 55B:117–123
King JR, Farner DS (1961) Energy metabolism, thermoregulation and body temperature. In: Marshal AJ (ed) Biology and comparative physiology of birds, 2nd edn. Academic Press Inc., London, pp 215–288
Lessells CM, Dingemanse NJ, Both C (2002) Egg weights, egg component weights, and laying gaps in Great Tits (Parus major) in relation to ambient temperature. Auk 119:1091–1103
Magrath RD (1992) Seasonal changes in egg-mass within and among clutches of birds: general explanations and a field study of the Blackbird Turdus merula. Ibis 134:171–179
Mänd R, Tilgar V, Leivits A (2000) Calcium, snails, and birds: a case study. Web Ecol 1:63–69
Massaro M, Davis LS (2004) The influence of laying date and maternal age on eggshell thickness and pore density in yellow-eyed penguins. Condor 106:496–505
Mazuc J, Bonneaud C, Chastel O, Sorci G (2003) Social environment affects female and egg testosterone levels in the house sparrow (Passer domesticus). Ecol Lett 6:1084–1090
McNeil KA, Newman I, Kelly FJ (1996) Testing research hypotheses with the general linear model. SIU Press, Illinois
Møller AP, Biard C, Blount JD, Houston DC, Ninni P, Saino N, Surai PF (2000) Carotenoid-dependent signals: indicators of foraging efficiency, immunocompetence or detoxification ability? Avian Poult Biol Rev 11:137–159
Møller AP, Karadas F, Mousseau TA (2008) Antioxidants in eggs of Great Tits Parus major from Chernobyl and hatching success. J Comp Physiol B 178:735–743
Morales J, Ruuskanen S, Laaksonen T, Eeva T, Mateo R, Belskii E, Ivankina EV, Järvinen A, Kerimov A, Korpimäki E, Krams I, Mänd R, Morosinotto C, Orell M, Qvarnström A, Siitari H, Slater FM, Tilgar V, Visser ME, Winkel W, Zang H, Moreno J (2013) Variation in eggshell traits between geographically distant populations of pied flycatchers Ficedula hypoleuca. J Avian Biol 44:111–120
Moreno J, Osorno JL (2003) Avian egg colour and sexual selection: does eggshell pigmentation reflect female condition and genetic quality? Ecol Lett 6:803–806
Moreno J, Lobato E, Morales J, Merino S, Tomás G, Martínez-de la Puente J, Sanz JJ, Mateo R, Soler JJ (2006) Experimental evidence that egg color indicates female condition at egg laying in a songbird. Behav Ecol 17:651–655
Moskát C, Avilés JM, Bán M, Hargitai R, Zölei A (2008) Experimental support for the use of egg uniformity in parasite egg discrimination by cuckoo hosts. Behav Ecol Sociobiol 62:1885–1890
Mousseau TA, Fox CW (1998) Maternal effects as adaptations. Oxford University Press, Oxford
Nager RG, van Noordwijk AJ (1992) Energetic limitation in the egg-laying period of Great Tits. Proc R Soc Lond B 249:259–263
Nager RG, Rüegger C, van Noordwijk AJ (1997) Nutrient or energy limitation on egg formation: a feeding experiment with Great Tits. J Anim Ecol 66:495–507
Nilsson J-Å, Svensson E (1993) Causes and consequences of egg mass variation between and within Blue Tit clutches. J Zool 230:469–481
Norris K (1993) Seasonal variation in the reproductive success of Blue Tits: an experimental study. J Anim Ecol 62:287–294
Ojanen M (1983a) Egg development and related nutrient reserve depletion in the Pied Flycathcer Ficedula hypoleuca. Annal Zool Fenn 20:293–300
Ojanen M (1983b) Effects of laying sequence and ambient temperature on the composition of eggs of the Great Tit Parus major and the Pied Flycatcher Ficedula hypoleuca. Annal Zool Fenn 20:65–71
Otto C (1979) Environmental factors affecting egg weight within and between colonies of Fieldfare Turdus pilaris. Ornis Scand 10:111–116
Pahl R, Winkler DW, Graveland J, Batterman BW (1997) Songbirds do not create long-term stores of calcium in their legs prior to laying: results from the high-resolution radiography. Proc R Soc B 264:239–244
Perrins CM (1970) The timing of birds’ breeding seasons. Ibis 112:242–255
Perrins CM (1996) Eggs, egg formation and the timing of breeding. Ibis 138:2–15
Pimentel E, Vidal LM, Cruces MP, Janczur MK (2013) Action of protoporphyrin-IX (PP-IX) in the lifespan of Drosophila melanogaster deficient in endogenous antioxidants, Sod and Cat. Open J Anim Sci 3:1–7
Remeš V, Matysioková B, Klejdus B (2011) Egg yolk antioxidant deposition as a function of parental ornamentation, age, and environment in Great Tits Parus major. J Avian Biol 42:387–396
Safran RJ, Pilz KM, McGraw KJ, Correa SM, Schwabl H (2008) Are yolk androgens and carotenoids in barn swallow eggs related to parental quality? Behav Ecol Sociobiol 62:427–438
Safran RJ, McGraw KJ, Pilz KM, Correa SM (2010) Egg-yolk androgen and carotenoid deposition as a function of maternal social environment in barn swallows Hirundo rustica. J Avian Biol 41:470–478
Saino N, Romano M, Ambrosini R, Ferrari RP, Møller AP (2004) Timing of reproduction and egg quality covary with temperature in the insectivorous barn swallow Hirundo rustica. Funct Ecol 18:50–57
Sanz JJ, García-Navas V (2009) Eggshell pigmentation pattern in relation to breeding performance of blue tits Cyanistes caeruleus. J Anim Ecol 78:31–41
Schifferli L (1973) The effect of egg weight on the subsequent growth of nestling Great Tit Parus major. Ibis 115:549–558
Senar JC, Polo V, Uribe F, Camerino M (2000) Status signalling, metabolic rate and body mass in the siskin: the cost of being subordinate. Anim Behav 59:103–110
Singer JD, Willett JB (2003) Applied longitudinal data analysis: modeling change and event occurrence. Oxford University Press, Oxford
Smith HG, Bruun M (1998) The effect of egg size and habitat on starling nestling growth and survival. Oecologia 115:59–63
Solomon SE (1997) Egg and eggshell quality. Iowa State University Press, Iowa
Stein LR, Badyaev AV (2011) Evolution of eggshell structure during rapid range expansion in a passerine bird. Funct Ecol 25:1215–1222
Stoddard MC, Marshall KLA, Kilner RM (2011) Imperfectly camouflaged avian eggs: artefact or adaptation? Avian Biol Res 4:196–213
Stoddard MC, Fayet AL, Kilner RM, Hinde CA (2012) Egg speckling patterns do not advertise offspring quality or influence male provisioning in great tits. PLoS ONE 7:e40211
Stokke BG, Moksnes A, Røskaft E, Rudolfsen G, Honza M (1999) Rejection of artificial cuckoo (Cuculus canorus) eggs in relation to variation in egg appearance among reed warblers (Acrocephalus scirpaceus). Proc R Soc Lond B 266:1483–1488
Styrksy JD, Eckerle KP, Thompson CF (1999) Fitness-related consequences of egg mass in nestling house wrens. Proc R Soc Lond B 266:1253–1258
Surai PF (2002) Natural antioxidants in avian nutrition and reproduction. Nottingham University Press, Nottingham
Surai PF, Noble RC, Speake BK (1999) Relationship between vitamin E content and susceptibility to lipid peroxidation in tissues of the newly hatched chick. Br Poult Sci 40:406–410
Taylor LR (1963) Analysis of the effect of temperature on insects in flight. J Anim Ecol 32:99–117
Tilgar V, Mänd R, Leivits A (1999) Effect of calcium availability and habitat quality on reproduction in Pied Flycatcher Ficedula hypoleuca and Great Tit Parus major. J Avian Biol 30:383–391
Török J (1986) Food segregation in three hole-nesting bird species during the breeding season. Ardea 74:129–136
Török J, Tóth L (1988) Density dependence in reproduction of the collared flycatcher (Ficedula albicollis) at high population levels. J Anim Ecol 57:251–258
Török J, Hargitai R, Hegyi G, Matus Z, Michl G, Péczely P, Rosivall B, Tóth Gy (2007) Carotenoids in the egg yolks of collared flycatchers (Ficedula albicollis) in relation to parental quality, environmental factors and laying order. Behav Ecol Sociobiol 61:541–550
Verboven N, Evans NP, D’Alba L, Nager RG, Blount JD, Surai PF, Monaghan P (2005) Intra-specific interactions influence egg composition in the lesser black-backed gull (Larus fuscus). Behav Ecol Sociobiol 57:357–365
Verhulst S, van Balen JH, Tinbergen JM (1995) Seasonal decline in reproductive success of the Great Tit: variation in time or quality? Ecology 76:2392–2403
Williams TD (1994) Intraspecific variation in egg size and egg composition in birds: effects on offspring fitness. Biol Rev 68:35–59
Williams TD (2012) Physiological adaptations for breeding in birds. Princeton University Press, Princeton
Wingfield JC (1994) Hormone-behavior interactions and mating systems in male and female birds. In: Short RV, Balaban E (eds) The Differences Between the Sexes. Cambridge University Press, Cambridge, pp 303–330
Woodall AA, Britton G, Jackson MJ (1997) Carotenoids and protection of phospholipids in solution or in liposomes against oxidation by peroxyl radicals: relationship between carotenoid structure and protective ability. Biochim Biophys Acta 1336:575–586
Acknowledgments
We are grateful to Gy. Blázi, N. Boross, L.Z. Garamszegi, G. Hegyi, D. Kiss, M. Laczi, G. Markó, B. Rosivall, and E. Szász for help with the fieldwork. We thank the Pilis Park Forestry for giving permission to erect nestboxes in the woodland area. We are very thankful for the helpful comments and suggestions of two anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding statement
This study was supported by the Hungarian Scientific Research Fund (OTKA, grants no. K75618 and PD100304), the Bolyai János Research Fellowship to R.H., and the Erdők a Közjóért Alapítvány.
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The Middle-Danube-Valley Inspectorate for Environmental Protection, Nature Conservation and Water Management provided permission for this study (KTVF 12677-4/2012).
Additional information
Communicated by L. Fusani.
Rights and permissions
About this article
Cite this article
Hargitai, R., Herényi, M., Nagy, G. et al. Effects of environmental conditions on the egg mass, yolk antioxidant level, eggshell thickness and eggshell spotting patterns of Great Tits (Parus major). J Ornithol 157, 995–1006 (2016). https://doi.org/10.1007/s10336-016-1348-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-016-1348-0