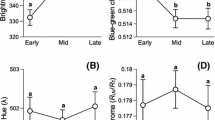
Abstract
The large variation in colouration and patterning of bird eggs suggests a variety of functions. For instance, in cases of intra- and inter-specific brood parasitism, the recognition of own eggs by the parents could be essential for their reproductive success. However, individual specific signatures may also be of interest from an applied point of view, as it would be possible to monitor individual females across breeding seasons by identifying their eggs. This would be of particular importance for species that are difficult to catch and ring such as the Common Crane (Grus grus). Since 2004, nest monitoring of this species has been conducted by one of us (W.M.) in north-east Germany, which led to the development of a semi-quantitative method to identify female cranes by diagnostic egg features including ground colour and spots of eggshells. In order to verify this approach, we quantitatively determined the spot patterns on eggshells from eggs of 19 females identified by this method. We used standardised photographs of the eggs laid across three seasons and the computer program “Egg Shell Pattern ANAlysis” (ESPANA). The resulting data were statistically analysed by conducting principal coordinate analyses and analyses of similarity. To prove the identity of the putative females, we extracted DNA for microsatellite analyses from eggshell pieces collected after hatching from up to seven breeding seasons. Our analyses confirmed that Common Cranes lay eggs with individual specific patterns and confirmed the reliability of the semi-quantitative method of identification. Microsatellite genotypes based on nine loci were identical for all samples from each particular, putative female. Therefore, the semi-quantitative approach of identifying females based on their clutches is indeed an innovative monitoring tool that will make many species accessible for addressing important issues in population biology, ecology and conservation.
Zusammenfassung
Genetischer Nachweis für individuelle Eierschalenfärbung von Kranichweibchen ( Grus grus )
Die Vielfalt in der Färbung und Zeichnung von Vogeleiern legt eine Reihe an möglichen Funktionen nahe. So könnte im Fall von intra- oder interspezifischem Brutparasitismus das Erkennen der eigenen Eier eine wichtige Rolle für den Fortpflanzungserfolg der Eltern spielen. Individuell spezifische Signaturen könnten zu dem von Interesse sein, wenn dadurch ein Monitoring von Weibchen währende der Brutzeit ermöglicht würde. Dies wäre von besonderer Bedeutung für die Arten, die nur schwer individuell unterschieden oder markiert werden können, wie beispielsweise den Grauen Kranich (Grus grus). Ein in Norddeutschland laufendes Brutmonitoring führte 2004 zur Entwicklung einer semi-quantitativen Methode, die die Identifizierung von Kranichweibchen anhand von Eiermerkmalen, wie der Eischalengrundfarbe und der Fleckenzeichung ermöglichte. Zur Verifizierung der Methode wurde die Eischalenmusterung von 19 durch sie individuell identifizierten Kranichweibchen quantitativ bestimmt. Grundlage waren standardisierte Fotografien von Eiern aus drei Brutperioden, die mit dem Computerprogramm „Egg Shell Pattern ANAlysis” (ESPANA) analysiert wurden. Die gewonnenen Daten wurden mittels einer Hauptkoordinatenanalyse (PCoA) und einer Ähnlichkeitsanalyse (ANOSIM) statistisch ausgewertet. Um die Identität der angenommen Kranichweibchen zu beweisen, extrahierten wir aus Eierschalenstücken, die nach dem Schlupf der Küken über mehrere Brutperioden gesammelt wurden, DNA für Mikrosatellitenanalysen. Unsere Analysen bestätigten, dass Kranichweibchen Eier mit individuell spezifischen Mustern legen, und damit auch die semi-quantitative Identifizierungsmethode. Die mittels neun Loci bestimmten Genotypen waren für die angenommenen Kranichweibchen über alle Proben identisch. Somit ist die semi-quantitative Methode zur Identifizierung von Kranichweibchen anhand ihrer Gelege ein innovatives Werkzeug zum Monitoring, um auch andere Arten für Fragen der Populationsbiologie, der Ökologie und des Naturschutzes zugänglich zu machen.


Similar content being viewed by others
References
Brulez K, Cassey P, Meeson A, Miksik I, Webber SL, Gosler AG, Reynolds SJ (2014) Eggshell spot scoring methods cannot be used as a reliable proxy to determine pigment quantity. J Avian Biol 45:94–102
Cassey P, Maurer G, Lovell PG, Hanley D (2011) Conspicuous eggs and colourful hypotheses: testing the role of multiple influences on avian eggshell appearance. Avian Biol Res 4(4):185–195
Cassey P, Hauber ME, Maurer G, Ewen JG (2012) Sources of variation in reflectance spectrophotometric data: a quantitative analysis using avian eggshell colours. Methods Ecol Evol 3:450–456
Cherry M, Gosler AG (2010) Avian eggshell colouration: new perspectives on adaptive explanations. Biol J Linn Soc 100:753–762
Cuthill IC (2006) Colour perception. In: Hill GE, McGraw KJ (eds) Bird colouration. Harvard University Press, Cambridge, pp 3–40
Dawson DA, Horsburgh GJ, Küpper C et al (2010) New methods to identify conserved microsatellite loci and develop primer sets of high cross-species utility—as demonstrated for birds. Mol Ecol Resour 10:475–494
Glenn TC, Ojerio RS, Stephan W, Braun MJ (1997) Microsatellite DNA loci for genetic studies of cranes. In: Urbanek RP, Stahlecker DW (ed) Proceedings of the 7th North American crane workshop, Lincoln, pp 36–45
Gosler AG, Barnett PR, Reynolds SJ (2000) Inheritance and variation in eggshell patterning in the Great Tit Parus major. Proc R Soc Lond B 267:2469–2473
Gosler AG, Higham JP, Reynolds SJ (2005) Why are birds’ eggs speckled? Ecol Lett 8:1105–1113
Gosler AG, Connora OR, Bonser RHC (2011) Protoporphyrin and eggshell strength: preliminary findings from a passerine bird. Avian Biol Res 4(4):214–223
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electronica 4:1–9
Hanley D, Grim T, Cassey P, Hauber ME (2015) Not so colourful after all: eggshell pigments constrain avian eggshell colour space. Biol Lett 11:20150087
Hasegawa O, Ishibashi Y, Abe S (2000) Isolation and characterisation of microsatellite loci in the red- crowned crane Grus japonensis. Mol Ecol 9:1677–1678
Henne E (1999) Sonografische Untersuchungen zur Paarbildung und Revierbindung beim Grauen Kranich (Grus grus L.). In: Prange H, Nowald G, Mewes W (ed) Proceedings of 3rd European crane workshop, Halle, pp 66–72
Hill GE, McGraw KJ (2006) Bird colouration, volume 2: function and Evolution. Harvard University Press, Cambridge
Kalinowski ST, Taper ML, Marshall TC (2007) Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol Ecol 16:1099–1106
Kennedy GY, Vevers HG (1976) Survey of avian eggshell pigments. Comp Biochem Physiol B 55:117–123
Kilner RM (2006) The evolution of egg colour and patterning in birds. Biol Rev 81:383–406
López-de-Hierro MDG, Moreno-Rueda G (2010) Egg-spot pattern rather than egg colour affects conspecific egg rejection in the house sparrow (Passer domesticus). Behav Ecol Sociobiol 64:317–324
Makatsch W (1974) Die Eier der Vögel Europas, vol 1. Neumann Verlag, Radebeul
Makatsch W (1976) Die Eier der Vögel Europas, vol 2. Neumann Verlag, Radebeul
Marshall TC, Slate J, Kruuk LEB, Pemberton JM (1998) Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol 7:639–655
Maurer G, Portugal SJ, Cassey P (2011) Review: an embryo’s eye view of avian eggshell pigmentation. J Avian Biol 42:494–504
Meise W (1992) Handbuch der Oologie, 1st edn. Akademie Verlag, Berlin
Mewes W, Modrow M (2010) „Brutparasitismus“beim Kranich Grus grus. Vogelwelt 131:103–105
Mewes W, Rauch M (2010) Die Identifizierung brütende Kranichweibchen Grus grus anhand ihrer Gelege. Vogelwelt 131:93–102
Mewes W, Rauch M (2012) Der Schlupferfolg von Kranichgelegen Grus grus in einem Untersuchungsgebiet in Mecklenburg-Vorpommern in den Jahren 2003 bis 2012. Vogelwelt 133:195–212
Mikhailov KE (1997) Avian eggshells: an atlas of scanning electron micrographs. BOC Occ Publ 3, Tring
Mikšík I, Holáň V, Deyl Z (1996) Avian eggshell pigments and their variability. Comp Biochem Physiol B 113(3):607–612
Moksnes A, Røskaft E, Rudolfsen G et al (2008) Individual female common cuckoos Cuculus canorus lay constant egg types but egg appearance cannot be used to assign eggs to females. J Avian Biol 39:238–241
Morales J, Kim SY, Lobato E, Merino S, Tomas G, Martínez de la Puente J, Moreno J (2010) On the heritability of blue-green eggshell colouration. J Evol Biol 23:1783–1791
Moreno J, Osorno JL (2003) Avian egg colour and sexual selection: does eggshell pigmentation reflect female condition and genetic quality? Ecol Lett 6:803–806
Pike TW (2011) Egg recognition in Japanese quail. Avian Biol Res 4:231–236
Prange H (1989) Der Graue Kranich. Die Neue Brehm-Bücherei 229. A. Ziemsen Verlag, Wittenberg Lutherstadt
Raymond M, Rousset F (1995) GENEPOP (version1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Rousset F (2008) Genepop’007: a complete reimplementation of the Genepop software for Windows and Linux. Mol Ecol Resour 8:103–106
Sanz JJ, Garcia Navas V (2009) Eggshell pigmentation pattern in relation to breeding performance of Blue Tits Cyanistes caeruleus. J Anim Ecol 78:31–41
Schmitz Ornés A, Herbst A, Spillner A, Mewes W, Rauch M (2014) A standardized method for quantifying eggshell spot patterns. J Field Ornithol 85(4):397–407
Sezer M, Tekelioglu O (2009) Quantification of Japanese Quail eggshell colour by image analysis. Biol Res 42:99–105
Spottiswoode CN, Stevens M (2010) Visual modeling shows that avian host parents use multiple visual cues in rejecting parasitic eggs. Proc Natl Acad Sci USA 107:8672–8676
Spottiswoode CN, Stevens M (2011) How to evade a coevolving brood parasite: egg discrimination versus egg variability as host defences. Proc R Soc Lond B 278:3566–3573
Stevens M (2011) Avian vision and egg colouration: concepts and measurements. Avian Biol Res 4:168–184
Stoddard MC, Stevens M (2010) Pattern mimicry of host eggs by the Common Cuckoo, as seen through a bird’s eye. Proc R Soc Lond B 277:1387–1393
Surmacki A, Kuczynski L, Tryjanowski P (2006) Eggshell patterning in the Red-backed Shrike Lanius collurio: relation to egg size and potential function. Acta Ornithol 41:145–151
Wessling B (2000) Individual recognition of cranes, monitoring and vocal communication analysis by sonography—a broad application of modern bioacoustic techniques. Presentation at the IV. European crane workshop, Verdun. http://typo2.chnabfich.de/index.php?id=149&L=0#
Wheelwright NT, Graff ES, Norris DR (2012) Relative consistency in size, shape, and colouration of Savannah Sparrow eggs within and between breeding seasons. Condor 114(2):412–420
Winter SW (1991) The demoiselle crane in the agricultural landscape of the Ukrainian Steppen zone. In: Proceedings 1987 international crane workshop, Baraboo, Wisconsin, pp 285–294
Acknowledgments
We are grateful to M. Luhn for his suggestions and constructive criticism to optimize the standard photographs of the eggs. We also thank M. Rauch for his technical and field support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Wink.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Höltje, H., Mewes, W., Haase, M. et al. Genetic evidence of female specific eggshell colouration in the Common Crane (Grus grus). J Ornithol 157, 609–617 (2016). https://doi.org/10.1007/s10336-015-1311-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-015-1311-5