Abstract
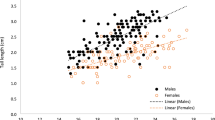
Both male and female Roseate Terns (Sterna dougallii) have unusually long outer tail feathers, and males tend to have longer tails than females. We examined whether these tail streamers may have evolved as a result of sexual selection, using data from a 15-year study at Bird Island, Massachusetts, USA. Data on tail length were analyzed for 2,515 terns, of which 745 were of known sex. Tail length was positively correlated with predictors of reproductive success, such as laying date, body mass, and age, and thus can act as an indicator of mate quality. The increase in mean tail length with age appeared to result from a combination of growth in relatively young terns and differential survival among older terns. The mean duration of pair bonds was short at 1.73 years. A female-biased sex ratio is present in this population, and we demonstrated that short-tailed females are not preferred mates: females paired to males had longer tails than those in female–female pairs or other multi-female associations. In male–female pairs, tail lengths of mates were correlated, but this may have resulted in part from the correlation in ages. These observations are consistent with the hypothesis that tail streamers are used by both sexes in mate choice. In contrast to our results for tail length, tail symmetry was not significantly related to indices of individual quality and was not significantly correlated between mates.
Zusammenfassung
Schwanzlänge und sexuelle Selektion in der monogamen und monomorphen Rosenseeschwalbe Sterna dougallii
Männliche wie weibliche Rosenseeschwalben (Sterna dougallii) haben ungewöhnlich lange äußere Schwanzfedern, wobei die der Männchen normalerweise noch länger sind. Anhand der Daten einer 15 jährigen Studie auf Bird Island in Massachusetts (USA) untersuchten wir, ob diese Schwanzfedern aufgrund sexueller Selektion entstanden. Wir untersuchten die Schwanzlängen von 2,515 Seeschwalben, wobei 745 bekannten Geschlechts waren. Schwanzlänge war positiv korreliert mit Variablen, die den Reproduktionserfolg beeinflussen (Legedatum, Körpermasse, Alter), und könnte daher als Anzeichen für die Qualität des Brutpartners verwendet werden. Das Wachstum junger Seeschwalben und ungleiche Überlebensraten älterer Individuen schien die Verlängerung der Schwanzfedern mit dem Alter zu bedingen. Die mittlere Dauer einer Paarbindung war 1,73 Jahre. In der Studienpopulation war ein Weibchenüberschuss gegeben und wir fanden, dass kurzschwänzige Weibchen nicht die bevorzugten Partner waren: Weibchen, die mit Männchen verpaart waren, hatten längere Schwanzfedern, als solche in Paaren von zwei Weibchen oder anderen Weibchenverbänden. In Männchen-Weibchen Paaren waren die Schwanzlängen der Partner korreliert, aber das mag zum Teil an der Korrelation der Alter von Paarpartnern gelegen haben. Diese Beobachtungen stimmen mit der Vermutung überein, dass die Länge der Schwanzfedern von beiden Geschlechtern in der Partnerwahl berücksichtigt wird. Entgegen der Ergebnisse für Schwanzlänge war die Symmetrie der Schwanzfedern kein Indiz für individuelle Qualität und war zwischen Partnern nicht signifikant korreliert.

Similar content being viewed by others
References
Amundsen T (2000) Why are female birds ornamented? Trends Ecol Evol 15:149–155
Andersson M (1982) Female choice selects for extreme tail length in a widowbird. Nature 299:818–820
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Arnold JM, Hatch JJ, Nisbet ICT (2004) Seasonal declines in reproductive success of the common tern Sterna hirundo: timing or parental quality? J Avian Biol 35:33–45
Bridge ES, Eaton MD (2005) Does ultraviolet reflectance accentuate a sexually selected signal in terns? J Avian Biol 36:18–21
Bridge ES, Nisbet ICT (2004) Wing molt and assortative mating in common terns: a test of the molt-signaling hypothesis. Condor 106:336–343
Burger J, Nisbet ICT, Safina C, Gochfeld M (1996) Temporal patterns in reproductive success in the endangered roseate tern (Sterna dougallii) nesting on Long Island, New York, and Bird Island, Massachusetts. Auk 113:131–142
Choudhury S, Black JM, Owen M (1992) Do barnacle geese pair assortatively? Lessons from a long-term study. Anim Behav 44:171–173
Clutton-Brock T (2009) Sexual selection in females. Anim Behav 77:3–11
Coulter MC (1986) Assortative mating and sexual dimorphism in the common tern. Wilson Bull 98:93–100
Cramp S, Simmons KEL (eds) (2004) BWPi: birds of the Western Palearctic interactive. (DVD-ROM). BirdGuides, Sheffield
Cuervo JJ, Møller AP (2000) Sex-limited expression of ornamental feathers in birds. Behav Ecol 11:246–259
Cuervo JJ, de Lope F, Møller AP (1996) The function of long tails in female Barn Swallows (Hirundo rustica): an experimental study. Behav Ecol 7:132–136
Cuervo JJ, Møller AP, de Lope F (2003) Experimental manipulation of tail length in female barn swallows (Hirundo rustica) affects their future reproductive success. Behav Ecol 14:451–456
Darwin C (1871) The descent of man, and selection in relation to sex. John Murray, London
Devlin CM, Diamond AW, Saunders GW (2004) Sexing arctic terns in the field and laboratory. Waterbirds 27:314–320
Evans MR, Hatchwell BJ (1992) An experimental study of male adornment in the scarlet-tufted malachite sunbird: II. The role of the elongated tail in mate choice and experimental evidence for a handicap. Behav Ecol Sociobiol 29:421–427
Fisher RA (1930) The genetical theory of natural selection. Clarendon, Oxford
Gangestad SW, Thornhill R (1999) Individual differences in developmental precision and fluctuating asymmetry: a model and its implications. J Evol Biol 12:402–416
Gochfeld M, Burger J, Nisbet ICT (1998) Roseate tern (Sterna dougallii). In: Poole A (ed) The birds of North America online. Cornell Laboratory of Ornithology, Ithaca. http://bna.cornell.edu/bna/species/370
González-Jaramillo M, de la Cueva H (2010) Natural tail streamer asymmetry in male magnificent frigatebirds Fregata magnificens: influence on mate selection and male parental care performance. Mar Ornithol 38:85–90
González-Solís J, Sokolov E, Becker PH (2001) Courtship feedings, copulations and paternity in common terns, Sterna hirundo. Anim Behav 61:1125–1132
Higgins PJ, Davies SJJF (eds) (1996) Handbook of Australian, New Zealand and Antarctic birds, vol 3: snipe to pigeons. Oxford University Press, Melbourne
Johnson K, Burley NT (1997) Mating tactics and mating systems of birds. In: Parker PG, Burley NT (eds) Avian reproductive tactics: female and male perspectives. Ornithol Monog 49, American Ornithologists’ Union, Washington, DC, pp 21–60
Jones IL, Hunter FM (1993) Mutual sexual selection in a monogamous seabird. Nature 362:238–239
Kirkpatrick M, Price T, Arnold SJ (1990) The Darwin–Fisher theory of sexual selection monogamous birds. Evolution 44:180–193
Kokko H, Johnstone RA (2002) Why is mutual mate choice not the norm? Operational sex ratios, sex roles and the evolution of sexually dimorphic and monomorphic signalling. Philos Trans R Soc Lond B 357:319–330
Kraaijeveld K, Kraaijeveld-Smit FJL, Komdeur J (2007) The evolution of mutual ornamentation. Anim Behav 74:657–677
Kvarnemo C, Ahnesjö I (1996) The dynamics of operational sex ratios and competition for mates. Trends Ecol Evol 11:404–408
Lande R (1980) Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34:292–305
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121
Limmer B, Becker PH (2007) The relative role of age and experience in determining variation in body mass during the early breeding career of the common tern (Sterna hirundo). Behav Ecol Sociobiol 61:1885–1896
Ludwig S, Becker PH (2008) Causes and consequences of assortative mating in common terns Sterna hirundo. Behav Ecol Sociobiol 62:1601–1611
Manning JT (1985) Choosy females and correlates of male age. J Theor Biol 116:349–354
Martinez-Padilla J, Vergara P, Pérez-Rodríguez L, Mougeot F, Casas F, Ludwig SC, Haines JA, Zeineddine M, Redpath SM (2011) Condition- and parasite-dependent expression of a male-like trait in a female bird. Biol Lett 7:364–367
Maynard Smith J (1991) Theories of sexual selection. Trends Ecol Evol 6:146–151
Møller AP (1988) Female choice selects for male sexual tail ornaments in the monogamous swallow. Nature 332:640–642
Møller AP, Höglund J (1991) Patterns of fluctuating asymmetry in avian feather ornaments: Implications for models of sexual selection. Proc R Soc Lond B 245:1–5
Møller AP, Swaddle JP (1997) Asymmetry, developmental stability and evolution. Oxford University Press, New York
Møller AP, Flensted-Jensen E, Mardal W (2007) Black beak tip coloration as a signal of phenotypic quality in a migratory seabird. Behav Ecol Sociobiol 61:1561–1571
Nisbet ICT, Hatch JJ (1999) Consequences of a female-biased sex-ratio in a socially monogamous bird: female–female pairs in the roseate tern Sterna dougallii. Ibis 141:307–320
Nisbet ICT, Spendelow JA, Hatfield JS, Zingo JM, Gough GA (1998) Variations in growth of roseate tern chicks: II. Early growth as an index of parental quality. Condor 100:305–315
Nisbet ICT, Bridge ES, Szczys P, Heidinger BJ (2007) Sexual dimorphism, female–female pairs, and test for assortative mating in common terns. Waterbirds 30:169–316
O’Donald P (1980) Genetic models of sexual and natural selection in monogamous organisms. Heredity 44:391–415
Palestis BG, Nisbet ICT, Hatch JJ, Szczys P, Spendelow JA (in press) Morphometric sexing of northwest Atlantic roseate terns. Waterbirds
Palmer AR (1994) Fluctuating asymmetry analyses: a primer. In: Markow T (ed) Developmental instability: its origins and evolutionary implications. Kluwer, Dordrecht, pp 335–364
Palmer AR, Strobeck C (2003) Fluctuating asymmetry analyses revisited. In: Polak M (ed) Developmental instability: causes and consequences. Oxford University Press, New York, pp 279–319
Parker GA (1983) Mate quality and mating decisions. In: Bateson P (ed) Mate choice. Cambridge University Press, Cambridge, pp 141–166
Regosin JV, Pruett-Jones S (2001) Sexual selection and tail-length dimorphism in scissor-tailed flycatchers. Auk 118:167–175
Rosvall KA (2011) Intrasexual competition in females: evidence for sexual selection? Behav Ecol 22:1131–1140
Rowe KMC, Weatherhead PJ (2011) Assortative mating in relation to plumage traits shared by male and female American robins. Condor 113:881–889
Sabo TJ, Kesseli R, Halverson JL, Nisbet ICT, Hatch JJ (1994) PCR-based method for sexing roseate terns (Sterna dougallii). Auk 111:1023–1027
Shealer DA, Cleary CM (2007) Sex determination of adult black terns by DNA and morphometrics: tests of sample size, temporal stability and geographic specificity in the classification accuracy of discriminant function models. Waterbirds 30:180–188
Szczys P, Nisbet ICT, Hatch JJ, Kesseli RV (2001) Sex ratio bias at hatching and fledging in the roseate tern. Condor 103:385–389
Thomas ALR, Balmford A (1995) How natural selection shapes birds’ tails. Am Nat 146:848–868
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man, 1871–1971. Aldine, Chicago, pp 136–179
US Fish and Wildlife Service (1987) Endangered and threatened wildlife and plants: determination of endangered and threatened status for two populations of the roseate tern. Fed Reg 25:42064–42071
Veit AC, Jones IL (2003) Function of tail streamers of red-tailed tropicbirds (Phaethon rubricauda) as inferred from patterns of variation. Auk 120:1033–1043
Velando A, Lessells CM, Márquez JC (2001) The function of female and male ornaments in the Inca tern: evidence for links between ornament expression and both adult condition and reproductive performance. J Avian Biol 32:311–318
Wendeln H (1997) Body mass of female common terns (Sterna hirundo) during courtship: relationships to male quality, egg mass, diet, laying date and age. Colon Waterbirds 20:235–243
Wendeln H, Becker PH (1999) Effects of parental quality and effort on the reproduction of common terns. J Anim Ecol 68:205–214
Winquist T, Lemon RE (1994) Sexual selection and exaggerated male tail length in birds. Am Nat 143:95–116
Acknowledgments
J. Spendelow coordinated studies of Roseate Terns at this and other sites and assisted with the 2009 measurements, and T. Sabo sexed most of the birds. We thank J. Spendelow, R. Trivers, P. Becker, C. Lessells, R. Ydenberg, and the referees for comments on previous versions of the manuscript, and C. Mostello, the Massachusetts Division of Fisheries and Wildlife, and the Town of Marion for logistic support and permission to work at Bird Island. Partial financial support was provided by the New Bedford Harbor Trustee Council, Massachusetts Audubon Society, Island Foundation, National Science Foundation (BIR-9322162), U.S. Fish and Wildlife Service, U.S. Geological Survey, and Wagner College. The procedures used were approved by the USGS-PWRC Animal Care and Use Committee and comply with the laws of the United States of America.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. H. Becker.
Rights and permissions
About this article
Cite this article
Palestis, B.G., Nisbet, I.C.T., Hatch, J.J. et al. Tail length and sexual selection in a monogamous, monomorphic species, the Roseate Tern Sterna dougallii . J Ornithol 153, 1153–1163 (2012). https://doi.org/10.1007/s10336-012-0846-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-012-0846-y