Abstract
Barley powdery mildew, caused by the obligate biotrophic fungus Blumeria graminis f. sp. hordei (Bgh), is one of the most devastating diseases in the winter barley-growing regions of China. Genetic diversity of this fungus was assessed by analyzing 75 Bgh isolates belonging to 12 pathotypes using seven primer combinations of amplified fragment length polymorphism (AFLP) markers. The six sampling locations were 300 km to 2200 km apart. The highest gene diversity indices were observed within Baoshan (H = 0.21) in Yunnan Province and Putian (H = 0.21) in Fujian Province. Higher gene flow values were found between Baoshan and other populations (Nm = 2.010−5.765) except Yancheng in Jiangsu Province and Jiaxing in Zhejiang Province and between Putian and other populations (Nm = 2.110–5.423) except Jiaxing though a long geographical distance among some locations. Between each Bgh population pair, the genetic groups and origins of the Bgh isolates were closely correlated, and the populations were significantly differentiated (P < 0.005 or P < 0.001). These results suggested that, in spite of local adaptation of Bgh populations to hosts, frequent long distance gene flow existed among these populations in China as shown in the previous study based on virulence assessment, and the centers of genetic diversity and primary inoculum origins of Bgh were probably located in those barley-growing areas in southwestern Yunnan Province and southeastern Fujian Province.
Similar content being viewed by others
Introduction
Powdery mildew, caused by the obligate biotrophic fungus Blumeria graminis f. sp. hordei (Bgh), is one of the most destructive diseases of barley in many major production regions worldwide (Dreiseitl and Kosman 2013; Jørgensen and Wolfe 1994). The disease is of great economic importance because it not only reduces yield but also lowers the quality of the harvested grain (Conry and Dunne 2001; Zhang et al. 2005). The genetic structure of Bgh populations changes constantly, and the level of pathogenic variability is dependent on many factors, such as the presence of resistance genes in the host, long distance spore dispersal, sexual recombination and mutation frequency (Caffier et al. 1999; Dreiseitl 2000; Dreiseitl and Wang 2007; Dreiseitl et al. 2006; Huang et al. 1995; Newton et al. 1998). During its epidemic outbreaks, Bgh frequently reproduces asexually on green leaves using conidiophores, which can be dispersed easily by wind. Thus, there is a high potential for gene flow among populations of the pathogen (Bousse and de Vallavieille-Pope 2003; Müller et al. 1996; Wolfe and McDermott 1994).
Sexual reproduction takes place when Bgh vegetative growth stops, and chasmothecia are produced during the summer on dry leaf debris. These chasmothecia usually release ascospores when winter barley is newly emerging in the autumn (Braun et al. 2002; Götz et al. 1996). The sexual stage increases genetic diversity and is a source of primary inoculum, especially in regions with hot summers. In contrast, sexually produced ascospores do not contribute greatly to the spread of powdery mildew (Bousse and de Vallavieille-Pope 2003; Limpert 1987).
Avirulence genes in the pathogen are matched by corresponding resistance genes in the host according to a gene-for-gene relationship (Jørgensen and Wolfe 1994). Because Bgh isolates adapt to barley resistance genes, virulence changes in the pathogen population are induced by direct selection. The genetic diversity of Bgh is very high in natural populations, especially in regions where the disease is a major problem (Andrivon and de Vallavieille-Pope 1993; Caffier et al. 1996; Dreiseitl 2003).
Breeding for disease resistance is an economically and environmentally sustainable way to protect barley from powdery mildew. Knowledge on the virulence and genetic diversity of the Bgh population is a prerequisite for the effective use of available resistance genes. Virulence assessments can provide useful information regarding virulence variation in Bgh populations and the effectiveness of host resistance genes. However, these assessments may only detect a limited number of virulence phenotypes because virulence types are subject to strong selection according to the gene-for-gene relation, and known genes in differential hosts are limited (Jin et al. 2014; Kolmer 1993; Leung et al. 1993; McDonald and McDermott 1993). In contrast, DNA fingerprinting techniques, which can detect a high level of allelic polymorphism in whole genomes, have exhibited great potential for studying genetic diversity in pathogen populations since their introduction in the 1990s (Péros et al. 2005; Ploetz et al. 2005; Wang et al. 2014). Molecular markers have been used to investigate the spread of clones bearing virulence and genetic diversity in Bgh populations (Brown 1994; Brown et al. 1991; Caffier et al. 1999; McDonald and McDermott 1993; Tucker et al. 2015).
China is one of the centers of barley domestication (Dai et al. 2012). Naturally grown wild barley is widely distributed in 44 counties between 27 and 32°N, 88 and 98°E from 1600 to 4350 m above sea level (Yu et al. 1998). Barley powdery mildew occurs widely and is more serious in winter-barley-growing regions, which are situated in southern China and represent more than half of the total barley production acreage in China. Although the virulence and genetic diversity of Bgh populations have been extensively investigated in countries in Europe (Bousse and de Vallavieille-Pope 2003; Dreiseitl et al. 2006; Müller et al. 1996), the genetic structure of Bgh populations has rarely been reported in China until recent years when the genetic diversity of Bgh populations in the winter-barley-growing regions of China has been examined using virulence phenotype assessment (Dreiseitl and Wang 2007; Zhu et al. 2010).
The amplified fragment length polymorphism (AFLP) technique has been increasingly used to characterize plant, nematode, bacterial and fungal populations genetically (Fargette et al. 2005; Fu and Williams 2008; Majer et al. 1996; Paul et al. 1997; Tooley et al. 2000). It has the merits of RFLP (restriction fragment length polymorphism) and RAPD (random amplified polymorphic DNA) and is more reproducible than RAPD (Vos et al. 1995; Zabeau and Vos 1993). In this paper, we used AFLP to investigate the molecular genetic structure of Bgh populations from China and to estimate the extent and range of gene flow among different geographical populations.
Materials and methods
B. graminis f. sp. hordei isolates and DNA extraction
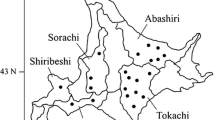
Seventy-five Bgh isolates were selected from ones collected in March to May 2006 before the peak incidence of powdery mildew development (Table 1, Zhu et al. 2009). Leaf samples were collected from different barley cultivars in different barley fields. The distance between two nearest collection sites in a field was at least 10 m apart. The Bgh isolates that were analyzed with AFLP came from six geographically distant locations: Baoshan (BS, Yunnan Province), Jiaxing (JX, Zhejiang Province), Jingzhou (JZ, Hubei Province), Putian (PT, Fujian Province), Yancheng (YC, Jiangsu Province), and Zhumadian (ZMD, Henan Province) (Fig. 1). These provinces are the most important barley-producing areas of the winter-barley-growing regions of China and are between 300 and 2200 km apart (Table 2). The isolates tested were selected from 16 barley cultivars and grouped into 12 pathotypes based on the virulence assessment of inoculations into ‘Pallas’ near-isogenic lines as differential hosts (Table 1, Zhu et al. 2009). Designation of the pathotypes is derived from their virulence patterns corresponding to 8 Ml resistance genes in coded triplets (Limpert et al. 1994) in the order: a7, a10, a12; a23, k1, at; La, h (Table 1, Zhu et al. 2009). To ensure that the samples detected were broadly representative, we selected isolates from different pathotypes and host varieties as many as possible, and more isolates included in some predominant virulence phenotypes were detected.
DNA extraction
Conidia of each were multiplied on leaf segments of the susceptible line Hua30 in 100 mm glass Petri dishes with 1.0 % (w/v) water agar and 35 mg/L benzimidazole (Dreiseitl and Wang 2007), then collected from the severely diseased segments in a cellophane bag by gentle shaking as described by Justesen et al. (2002). Total genomic DNA was extracted from the spores following a modified version of the CTAB-proteinase K method (Godwin et al. 1997) as follows: conidia (10–20 mg) were dried in a drying apparatus with a solid desiccant for 3 days, then pulverized by vortexing in a 2 mL tube containing 11 (3 mm diameter) glass beads and 200 mL of extraction buffer (25 g/L d-sorbitol, 10 g/L N-lauroylsarcosine, 8 g/L cetyltrimethylammonium bromide, 47 g/L NaCl, 8 g/L EDTA, 0.1 M Tris, pH 8.0) and heated to 65 °C for 2.5 min. Then, 300 mL of extraction buffer containing 5 mL of proteinase K (10 mg/mL) was added to the vortex tubes.
After 1.5 h at 65 °C, the spore suspension was gently mixed with 0.5 mL chloroform and centrifuged at 4 °C for 20 min at 1.5 × 104×g. Three hundred microliters of the supernatant was transferred to a 1.5 mL tube and incubated at −20 °C for 10 min with 0.5 mL isopropanol. DNA was precipitated by centrifuging for 20 min at 2.0 × 104 × g, rinsed with 70 % (v/v) ethanol, air dried and incubated in 0.2 mL 0.1 × TE buffer (10 mM Tris pH 8.0, 1 mM EDTA) containing 1 µL RNase (10 mg/mL) at 37 °C for 1 h. Finally, the DNA was stored in 20 µL TE buffer (10 mM Tris pH 8.0, 1 mM EDTA) at −20 °C for later use. DNA was quantified in 1 % (w/v) agarose gels.
AFLP analysis
AFLP analysis of the Bgh isolates followed the protocol of Vos et al. (1995) with minor modifications. Genomic DNA (200 ng) was digested doubly using the restriction enzymes EcoRI and MseI. Following heat inactivation of the restriction endonucleases, EcoRI adapters (adp1: 5′-CTCGTAGACTGCGTACC-3′, adp2: 5′-AATTGGTACGCAGTCTAC-3′) and MseI adapters (adp1: 5′-GACGATGAGTCCTGAG-3′, adp2: 5′-TACTCAGGACTCAT-3′) were ligated to the DNA fragments overnight at 16 °C to generate template DNA. The digestion–ligation mixtures were diluted tenfold before being used as templates for pre-amplification. Template DNA was first pre-amplified using the primers EcoRI and MseI without the use of selective nucleotides. The pre-amplification cycling protocol was as follows: 25 cycles of denaturation at 94 °C for 30 s, annealing at 52 °C for 30 s and incubation at 72 °C for 1 min followed by a final extension at 72 °C for 7 min. The pre-amplification products were diluted tenfold and then used as templates for selective amplification with two primers, each containing two or three selective nucleotides (Table 3).
To obtain an optimum number of scored polymorphic bands, 112 EcoRI + 2/MseI + 3 or EcoRI + 3/MseI + 2 primer combinations were tested, and seven primer EcoRI + 2/MseI + 3 or EcoRI + 3/MseI + 2 pairs were selected (Table 3). Selective amplification was performed as follows: one cycle of 94 °C for 30 s, 62 °C for 30 s and 72 °C for 1 min; followed by 11 cycles, decreasing the annealing temperature by 0.7 °C each cycle, starting with 94 °C for 30 s, 62 °C for 30 s and 72 °C for 1 min; followed by 22 cycles of 94 °C for 30 s, 54 °C for 30 s and 72 °C for 1 min; with a final extension at 72 °C for 7 min. After the amplification products were mixed with loading buffer and denatured by heating, the DNA fragments were separated using 6 % polyacrylamide gels and visualized by silver staining (Zhong and Steffenson 2000).
Data analysis
AFLP bands that were present in some isolates but absent in other isolates were considered polymorphic markers. The presence and absence of a polymorphic marker were scored 1 and 0, respectively. From polymorphic AFLP bands for each isolate, a binary presence (1)/absence (0) matrix was constructed. Data matrices were analyzed using the Treecon program, version 1.3b (van de Peer and de Wachter 1994). Genetic similarities were calculated based on Jaccard’s coefficient. Cluster analysis performed on the similarity matrices generated using the UPGMA (unweighted pair group method with arithmetic average) algorithm, and a dendrogram was created with the DRAW option of Treecon (Nelson et al. 1994). The Mantel test (Mantel 1967) of correlation between the genetic similarity matrixes of virulence phenotypes and molecular marker clusters was done with MXCOMP in NTSYS-pc V2.1 (Exeter Software, Setauket, NY). The AFLP marker data set was analyzed with a principal coordinate analysis (PCA) and a 3D scatter plot constructed using NTSYS-pc V2.1. Gene diversity (H) and Nei’s unbiased genetic distance were calculated using the program POPGEN version 1.32 (available via DIALOG. http://www.ualberta.ca/~fyeh/popgene down-load.html. Cited 8 Dec 2014). POPGEN was also used to test allele association among loci (Brown et al. 1980). The correlation analysis between genetic distance and geographical distance of strains from six sampling sites was conducted using the Mantel test procedure implemented in IBD 1.52 (Bohonak 2002). Significance was assessed based on 1000 randomizations. Analysis of molecular variance (AMOVA) and the determination of pairwise population genetic differentiation (F ST) and gene flow (Nm) were calculated using the program Arlequin 3.1 (Excoffier et al. 2005). Pairwise F ST distance estimates were calculated based on the number of different alleles, and the statistical significance of pairwise F ST estimates was assessed using a permutation test with 1000 permutations.
Results
AFLP cluster analysis of B. graminis f. sp. hordei populations
The seven primer combinations produced a total of 384 amplified bands from the 75 Bgh isolates, and 332 were polymorphic (86.46 %). The number of polymorphic bands per primer pair ranged from 35 to 64, with an average of 47.43 (Table 3). Seventy-five different AFLP phenotypes were observed when the AFLP markers generated by all primer pairs were combined. A dendrogram for all Bgh isolates was generated by Jaccard’s similarity matrix and the UPGMA method based on 332 AFLP markers. Considering that 75 Bgh isolates tested were grouped in 12 pathotypes based on their virulence phenotypes, we separated the AFLP dendrogram into 13 genetic groups at 85.4 % DNA similarity (Fig. 2). Most (81.33 %) of the isolates were included in five of the genetic groups (GP01, GP02, GP04, GP05, and GP09), and 18.67 % were included in the remaining eight groups. Five genetic groups (GP06, GP07, GP10,GP12, and GP13) were represented by a single isolate each. Within the populations, three predominant groups (GP01, GP02, and GP09) contained 32.00, 17.33, and 13.33 % of the isolates, respectively.
Dendrogram of 75 isolates of Blumeria graminis f. sp. hordei revealed by cluster analysis using the unweighted pair group method with arithmetic averages. Filled triangle Baoshan (BS) sampling site, filled diamond Jingzhou (JZ) sampling site, unfilled diamond Zhumadian (ZMD) sampling site, unfilled triangle Putian (PT) sampling site, filled star Yancheng (YC) sampling site, unfilled star Jiaxing (JX) sampling site, epathotype, fnumber of isolates is in parentheses
Isolates belonging to the same pathotypes were distributed among different AFLP genetic groups (Fig. 2; Table 4). For instance, the 14 isolates belonging to pathotype 003 were found in eight genetic groups except for GP03, GP06, GP07, GP12, and GP13, the 12 isolates in pathotype 010 were found in seven genetic groups except for GP04, GP06, GP07, GP10, GP11, and GP13, the 15 isolates in pathotype 000 were found in GP01, GP02, GP04, GP05, GP7, and GP8, and the 11 isolates in pathotype 001 were found in GP01, GP02, GP03, and GP08, respectively. In a correlation analysis based on the genetic similarity matrices for virulence and AFLP data, the correlation coefficient between the virulence phenotypes and AFLP banding patterns of 75 Bgh isolates was low and significant (r = −0.097, P < 0.05).
Most of the Bgh isolates collected from individual locations, except PT, were primarily included in one or two AFLP genetic groups (Fig. 2; Table 4). For example, 75.00 % of the isolates from BS and 65.00 % of the ones from ZMD were included in groups GP01 and GP02 respectively, each half of all the isolates from JZ in GP01 and GP05, all the isolates from YC in GP09 (62.50 %) and GP04 (37.50 %), 75.00 % of all the isolates from JX in GP09 (37.50 %) and GP11 (37.50 %). Some isolates from a location only belonged to a matching genetic group (Fig. 2; Table 4). For example, GP02 only contained 13 isolates from ZMD, and GP11 only contained three isolates from JX. Also, most of isolates clustered in the same genetic groups were distributed in some closer sampling sites. The PCA of 75 Bgh isolates based on AFLP markers show that most of the isolates from the same locations were clustered together in a Mod3D plot (Fig. 3).
Principal coordinate analysis (PCA) of 75 isolates of Blumeria graminis f. sp. hordei based on AFLP markers. Filled triangle Isolates from Baoshan (BS), filled diamond isolates from Jingzhou (JZ), unfilled diamond isolates from Zhumadian (ZMD), unfilled triangle isolates from Putian (PT), filled star isolates from Yancheng (YC), unfilled star isolates from Jiaxing (JX)
Molecular diversity and linkage disequilibrium
AFLP diversity parameters within and between six populations were tested with POPGEN version 1.32 (Table 5). The number of polymorphic loci within six different populations ranged from 174 to 256, and the proportion of polymorphic loci ranged from 45.31 to 66.67 %. Nei’s average gene diversity index (H) was 0.25 for the entire population of 75 Bgh isolates. The H value differed among populations (0.16–0.21) (P < 0.001); the BS (H = 0.21), JZ (H = 0.20) and PT populations (H = 0.21) had higher gene diversity indexes, and the ZMD (H = 0.18), YC (H = 0.17) and JX populations (H = 0.16) had lower gene diversity indexes. In all the populations, the observed variance V O was higher than the estimated 95 % cut-off value for significance L, indicating that significant linkage disequilibrium exists among the AFLP loci within these populations. AMOVA was used to assess overall variation within and among populations, and 80.51 % of the total genetic diversity was found within populations, and 19.49 % of the diversity was found among populations (F ST = 0.195, P < 0.001).
Genetic differentiation and gene flow
Significant population differentiation was found between each population pair, with F ST values ranging from 0.080 (BS and JZ) to 0.392 (ZMD and JX) (Table 6). The pairwise population gene flow (Nm) across six populations was from 0.775 (ZMD/JX) to 5.765 (BS/JZ) (Table 6). High Nm values (Nm > 4) were found between JZ and BS (Nm = 5.765) or PT (Nm = 5.423), and between PT and YC (Nm = 4.820). Higher Nm values were found between BS and PT (Nm = 3.480) or ZMD (Nm = 2.010), between JZ and ZMD (Nm = 3.337), between ZMD and PT (Nm = 2.110), and between PT and JX (Nm = 2.070). Low Nm values were found between YC (Nm = 1.065–1.636) or JX (Nm = 0.775–1.636) and other locations except PT. Pairwise estimates of Nei’s genetic distance detected between populations ranged from 0.045 (BS/JZ) to 0.199 (BS/JX) (Table 2). Based on the matrix, the Mantel test revealed that no significant positive correlation (r = 0.1707, P = 0.249) existed between genetic distance and geographical distance between populations, and no evidence of isolation by distance was found.
Discussion
Genetic diversity of Bgh populations
In the study, a cluster analysis showed that 13 AFLP genetic groups among 75 Bgh isolates were classified at 85.4 % similarity, and the gene diversity (H) for all populations was 0.25, providing further evidence that the Bgh populations in the winter-barley-grown regions of China exhibited greater genetic variation as shown in a previous study based on virulence assessment (Zhu et al. 2010). Indices of gene diversity for Bgh populations from BS (H = 0.21), PT (H = 0.21) and JZ (H = 0.20) were higher than ZMD (H = 0.18), YC (H = 0.17), and JX (H = 0.16), possibly because barley powdery mildew in BS, PT, and JZ is generally more serious than in other regions; larger areas are cultivated in barley and environmental conditions are more suited for propagation and survival in the hot summer (Dong et al. 2012; Huang et al. 2008; Zheng et al. 2013); thus mutation and sexual recombination are more frequent in the Bgh populations. In a previous study (Zhu et al. 2010) based on virulence evaluation, lower genetic diversity was detected in the Bgh population from JX and YC as shown in the present study, but lower genetic diversity was also found in the Bgh population from BS and PT. This result suggests that genetic variation can be estimated more accurately with molecular markers in contrast to virulence assessment because molecular markers can screen a greater number of putatively neutral genetic loci.
Gene flow and genetic differentiation between pairwise Bgh populations
The combination of a uniform agro-ecosystem and efficient inoculum dispersal may result in the observed genetic homogeneity, but geographical isolation and local adaptation can lead to genetic differentiation. Barley powdery mildew undergoes multiple secondary cycles of asexual conidial reproduction during each growing season, and conidia can easily spread by wind (Wolfe and McDermott 1994). As temperatures rise at the end of the growing season, compatible mating types can intercross randomly (Braun et al. 2002), so gene exchange may be frequent among different populations. Brown and Hovmøller (2002) reported spores of the pathogen can move more than 2000 m above sea level by airflow and are wind-dispersed for hundreds of kilometers. Hermansen et al. (1978) demonstrated that viable barley mildew spores could disperse across the North Sea from Britain to Denmark, a distance of ≈800 km. Brown (1994) reported that Bgh isolates with novel virulences were transmitted long distances from continental Europe to Britain.
In general, if the Nm value between a population pair is more than 4, gene flow is frequent and can even counteract genetic drift to lead to genetic differentiation (Whitlock and McCauley 1999). Here, very high gene flow levels (Nm > 4) were found between BS and JZ (Nm = 5.765) and between PT and JZ (Nm = 5.423) or YC (Nm = 4.820), and high Nm values were detected between BS and PT (Nm = 3.480) or ZMD (Nm = 2.010) and between PT and ZMD (Nm = 2.110). However, the geographical distances between BS and JZ (1360 km), ZMD (1670 km) or PT (1950 km) are very great, and even greater between PT and JZ (860 km), ZMD (970 km) or YC (900 km). Low Nm values among some adjacent populations, such as between YC and JX (Nm = 1.636) and between ZMD and YC (Nm = 1.065) or JX (Nm = 0.775), were found. The distance between these population pairs is from 300 to 680 km. AMOVA analysis proved that most of the overall genetic diversity was partitioned within populations (80.51 %). The Mantel test showed that this pattern of gene flow was inconsistent with the model of isolation by distance. These results suggested that there was frequent long distance migration among some populations and that the migration could be finished by one step. Significant linkage disequilibrium was found with the POPGEN analysis, suggesting that asexual reproduction in these populations is predominant and asexually produced conidiophores contribute greatly to the spread of powdery mildew.
In the Chinese winter-barley-growing regions with a subtropical monsoon climate, southeast and southwest winds prevail from April to August and north and northwest winds do from September to March next year (Chen and Lu 1981), so Bgh spores can disperse by wind over long distances among these different locations. BS is located on the southwest Yunnan-Guizhou Plateau high above sea level, where the temperature is relatively high in the winter and low in the summer and the wind direction is almost southwest all year (You 2001). Thus, Bgh conidia on host leaves can reproduce abundantly with appropriate humidity and be transported continuously to the northeast by wind. Moreover, at PT on the southeast coast of China, the mean temperature has increased rapidly, and there is quite a lot of rain in the spring, so barley powdery mildew occurs early and frequently (Chen et al. 1996), and huge numbers of Bgh spores can disperse via a prevailing southeast wind. As mentioned already, higher gene flow values mostly were found between BS or PT and other populations, although BS or PT was much farther from these locations. Interestingly, the highest gene flows were found between JZ and BS (Nm = 5.765) or PT (Nm = 5.423), perhaps because JZ is at the closest junction of the two airflows from southwest BS and southeast PT. Genetic differentiation among Bgh populations is affected by many factors such as migration, mutation, sexual recombination, host selection and genetic drift. Obviously, the reason for genetic homogeneity among Bgh populations in the winter-barley-growing regions of China was largely explained by migration of Bgh spores from BS and PT. The highest genetic diversity index (H = 0.21) was detected in BS and PT. These results indicate that the center of genetic diversity and origin of primary inoculum of Bgh were probably located in those barley-growing areas in southwestern Yunnan Province and southeastern Fujian Province. But for convincing proof of this conclusion, Bgh isolates from more sites in these regions must be collected and analyzed.
Different gene flow levels among some populations can be partly explained by local adaption such as host selection, genetic drift and ecological condition. For example, ZMD is close to JZ (340 km), but Nm values between BS or PT and ZMD are much lower than between BS or PT and JZ. Likewise, much higher Nm values between PT (Nm = 4.820)and JX than PT and YC (Nm = 2.070) were found even with the very close geographic distance between JX and YC (300 km). Zhu et al. (2009) reported that some planted barley varieties in areas such as Yunnan, Henan, Jiangsu, Fujian and Zhejiang Provinces were resistant to some predominant Bgh pathotypes, indicating that the genetic changes in some populations could be caused partly through the selection of the local population responding to resistance genes in particular cultivars. Lower gene flow levels were found between YC or JX and other populations except PT. The most important cause for this result may be founder effects, which were followed by genetic drift caused by a marked seasonal reduction of local pathogen populations resulting from a long period of high temperature not conducive to Bgh prevalence over summer. This idea supports the proposal suggested in a previous study based on virulence assessment (Dreiseitl and Wang 2007; Zhu et al. 2010).
The six populations exhibited higher and significant differentiation (F ST) in all comparisons (F ST = 0.080–0.392, P < 0.001 or P < 0.05), and a cluster analysis based on AFLP polymorphism shows that the genetic groups are related to the geographical origin of the isolates, suggesting that the whole Bgh population is not a single metapopulation and that the Bgh isolates from each of the six locations forms a relatively separate geographical population by completing a life cycle of infection in 1 year at its respective location, though long-distance gene flow among these locations is frequent. Also, Enjalbert et al. (2005) found that the genetic diversity in French population of Puccinia striiformis f. sp. tritici, analyzed by microsatellite and AFLP markers, presented a strong geographical structure despite the long distance dispersal capacity of its spores. All this evidence supports that the genetic variation in Bgh populations from the winter-barley-growing regions of China was primarily derived from local evolution and long distance spread, in agreement with the conclusion we previously drew based on virulence diversity indexes (Zhu et al. 2010).
The relationship between genetic groups and pathotypes
Some isolates with different pathotypes were categorized into the same AFLP genetic lineage, for instance, the isolates that clustered into GP01 and GP02 included 10 and 8 different pathotypes, respectively (Table 4). There was no correlation between isolate AFLP groups and their virulence patterns, and a correlation analysis based on the genetic similarity matrices for AFLP and virulence data showed that the AFLP groups was not relative to the virulence patterns significantly (r = −0.097, P < 0.05). Caffier et al. (1999) found that groups of Bgh isolates on the basis of virulence assessment had different haplotypes defined by RAPD. This finding was also reported in previous studies on other fungi (Thuan et al. 2006; Zhong and Steffenson 2001). A possible explanation for the low correlation between virulence and genetic patterns based on molecular markers is that the evolution of the pathotype is independent of the evolution of neutral DNA markers (Wang et al. 2014), and genetic recombination in sexual reproduction may also contribute to new genotypes with a high degree of variation in virulence (Zhong and Steffenson 2001). In contrast, isolates of the same pathotype in some asexual populations may have greater genetic similarity than isolates from different pathotypes due to their isolated clonal reproduction. Caffier et al. (1999) reported the Bgh isolates belonging to the V22 pathotype in France were grouped into the same haplotype due to the survival of asexual lineages in populations that often reproduce sexually. Similar results were also observed in other asexually reproducing populations such as Fusarium oxysporum f. sp. vasinfectum (Asigbetse et al. 1994), Magnaporthe oryzae (Levy et al. 1993), Puccinia recondita (Kolmer et al. 1995) and Cylindrocarpon liriodendri (Alaniz et al. 2009). But de Jonge et al. (2013) reported that 11 strains of the strictly asexual pathogen Verticillium dahlia differed in virulence in tomato in spite of a high degree of identity (99.98 %) among their genomes and suggested that extensive chromosomal rearrangements act as a source of genetic variation and lead to differential virulence. Similar results were also found for Verticillium dahliae isolates (Gharbi et al. 2015). In our present study, some Bgh isolates clustered into the same AFLP genetic groups at a high similarity from the same locations and hosts, for example, X28 and X32 in G01, N09 and N11 in G02, M06 and M21 in G02, G08 and G09 in G09 (Fig. 2), and might be clones of a single strain (Bhat and Subbarao 1999), but the virulence was diverse among these isolates, which can be explained with a chromosomal reshuffling mechanism.
This study shows that Bgh populations in China have a high level of genetic diversity, with frequent long distance gene flow among the populations as shown in a previous study based on virulence assessment (Zhu et al. 2010). However, the levels of gene diversity (H) in Bgh populations detected with AFLP are possibly more accurate, suggesting molecular markers may be more useful than virulence phenotypes for analyzing the genetic structure of populations. Virulence assessment is of limited use in inferring population structure and offers no insights into historical structuring processes, with virulence pattern identification depending on host genotype and there being geographic variability in the deployment of resistance genes. Virulence assessment, however, can provide immediate information on the effectiveness of known resistance genes in host varieties. Besides AFLPs, many other molecular markers such as SSR, ISSR and SNP have been used to assess the genetic structure of barley and wheat powdery mildew populations (Jia et al. 2007; Parks et al. 2009; Tucker et al. 2015). Compared with SSR and ISSR, AFLP provides many more polymorphic bands, but the technique is more complicated and time-consuming and requires more technical expertise. However, the level of variation detected may deviate from the true level of variation at the nucleotide level with these marker systems because shared bands do not necessarily indicate common ancestry. Single-nucleotide polymorphisms (SNPs) in some housekeeping gene sequences can be utilized in more complex population genetic models to draw statistical inferences about historical processes structuring modern populations (Parks et al. 2009; Rosenberg and Nordborg 2002), but this molecular marker system can only investigate limited loci. Therefore, using appropriate molecular methods on basis of virulence assessment, more accurate inferences can be made concerning contemporary and historical processes that structured the barley powdery mildew population to help deploy useful resistance genes against powdery mildew in barley breeding.
References
Alaniz S, Armengol J, León M, Garcia-Jiménez J, Abad-Campos P (2009) Analysis of genetic and virulence diversity of Cylindrocarpon liriodendri and C. macrodidymum associated with black foot disease of grapevine. Mycol Res 113:16–23
Andrivon D, de Vallavieille-Pope C (1993) Racial diversity and complexity in regional populations of Erysiphe graminis f. sp. hordei in France over a 5-year period. Plant Pathol 42:443–464
Asigbetse KB, Fernandez D, Dubois MP, Geiger JP (1994) Differentiation of Fusarium oxysporum f. sp. vasinfectum races on cotton by random amplified polymorphic DNA (RAPD) analysis. Phytopathology 84:622–626
Bhat RG, Subbarao KV (1999) Host range specificity in Verticillium dahliae. Phytopathology 89:1218–1225
Bohonak AJ (2002) IBD (isolation by distance): a program for analyses of isolation by distance. J Hered 93:153–154
Bousse L, de Vallavieille-Pope C (2003) Effect of sexual recombination on pathotype frequencies in barley powdery mildew populations of artificially inoculated field plots. Eur J of Plant Pathol 109:13–24
Braun U, Cook RTA, Inman AJ, Shin HD (2002) The taxonomy of the powdery mildew fungi. In: Bélanger RR, Bushnell WR, Dik AJ, Carver TLW (eds) The powdery mildews: a comprehensive treatise. APS Press, St Paul, pp 13–55
Brown JKM (1994) Chance and selection in the evolution of barley mildew. Trends Microbiol 2:470–475
Brown JKM, Hovmøller MS (2002) Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science 297:537–541
Brown AHD, Fieldman MW, Nevo E (1980) Multilocus structure of natural populations of Hordeum spontaneum. Genetics 96:523–536
Brown JKM, Jessop AC, Rezanoor HN (1991) Genetic uniformity in barley and its powdery mildew pathogen. Proc R Soc Lond B 246:83–90
Caffier V, de Vallavieille-Pope C, Brown JKM (1996) Segregation of avirulences and genetic basis of infection types in Erysiphe graminis f. sp. hordei. Phytopathology 86:1112–1121
Caffier V, Brändle UE, Wolfe MS (1999) Genotypic diversity in barley powdery mildew populations in northern France. Plant Pathol 48:582–587
Chen J, Lu D (1981) Division of monsoon climatic regions in China. J Beijing Forest Univ 4:1–8
Chen B, Huang J, Guo Y, Chen D, Jiang W, Zhou Z (1996) Breeding of ‘Pudamai 5′ and study on the breeding way for resistance to powdery mildew of barley. J Fujian Acad Agr Sci 11:10–13
Conry MJ, Dunne B (2001) Influence of number and timing of fungicide applications on the yield and quality of early and later-sown spring malting barley grown in the south-east of Ireland. J Agr Sci 136:159–167
Dai F, Nevo E, Wu D, Comadran J, Zhou M, Qiu L, Chen Z, Beiles A, Chen G, Zhang G (2012) Tibet is one of the centers of domestication of cultivated barley. Proc Natl Acad Sci USA 109:16969–16973
de Jonge R, Bolton MD, Kombrink A, Van den Berg GCM, Yateda KA, Thomma BPHI (2013) Extensive chromosomal reshuffling drives evolution of virulence in an asexual pathogen. Genome Res 23:1271–1282
Dong J, Li M, Xu F, Qin D, Zhang J, Ge S (2012) Development trend and evolution analysis of barley varieties in Hubei. Barley Cereal Sci 4:1–4
Dreiseitl A (2000) Direct selection in the Blumeria graminis f. sp. hordei population in the Czech Republic. Acta Phytopathol Entomol Hun 35:317–322
Dreiseitl A (2003) Adaptation of Blumeria graminis f. sp. hordei to barley resistance genes in the Czech Republic in 1971–2000. Plant Soil Environ 49:241–248
Dreiseitl A, Kosman E (2013) Virulence phenotypes of Blumeria graminis f. sp. hordei in South Africa. Eur J Plant Pathol 136:113–121
Dreiseitl A, Wang J (2007) Virulence and diversity of Blumeria graminis f. sp. hordei in east China. Eur J Plant Pathol 117:357–368
Dreiseitl A, Dinoor A, Kosman E (2006) Virulence and diversity of Blumeria graminis f. sp. hordei in Israel and in the Czech Republic. Plant Dis 90:1031–1038
Enjalbert J, Duan X, Leconte M, Hovmølle S, de Vallavieille-Pope C (2005) Genetic evidence of local adaptation of wheat yellow rust (Puccinia striiformis f. sp. tritici) within France. Mol Ecol 14:2065–2073
Excoffier L, Laval G, Schneider S (2005) arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol Bioinform 1:47–50
Fargette M, Lollier V, Phillips M, Blok V, Frutos R (2005) AFLP analysis of the genetic diversity of Meloidogyne chitwoodi and M. fallax major agricultural pests. C R Biol 328:455–462
Fu Y, Williams DJ (2008) AFLP variation in 25 Avena species. Theor Appl Genet 117:333–342
Gharbi Y, Trikic MA, Trabelsi R, Fendri I, Daayf F, Gdoura R (2015) Genetic structure of Verticillium dahliae isolates infecting olive tree in Tunisia using AFLP, pathogenicity and PCR markers. Plant Pathol 64:871–879
Godwin ID, Aitken EAB, Smith LW (1997) Application of inter simple sequence repeat (ISSR) markers to plant genetics. Electrophoresis 18:1524–1528
Götz M, Friedrich S, Boyle C (1996) Development of cleistothecia and early ascospore release of Erysiphe graminis DC. f. sp. tritici in winter wheat in relation to host age and climatic conditions. J Plant Dis Protect 103:134–141
Hermansen JE, Torp U, Prahm LP (1978) Studies of transport of live spores of cereal mildew and rust fungi across the North Sea. Grana 17:41–46
Huang R, Kranz J, Welz HG (1995) Virulence gene frequency change in Erysiphe graminis f. sp. hordei due to selection by non-corresponding barley mildew resistance genes and hitchhiking. J Phytopathol 143:287–294
Huang J, Li Q, Chen H (2008) Identification and application of barley germplasm resources with resistance to powdery mildew. J Plant Gen Resour 9:101–104
Jia S, Duan X, Zhou Y, Lu G, Wang Z (2007) Establishment of ISSR PCR reaction system for Blumeria graminis f. sp. tritici and its application in diversity analysis of this pathogen. Acta Phytopathol Sinica 34:493–499
Jin Y, Rouse M, Groth J (2014) Population diversity of Puccinia graminis is sustained through sexual cycle on alternate hosts. J Integr Agr 13:262–264
Jørgensen JH, Wolfe M (1994) Genetics of powdery mildew resistance in barley. Crit Rev Plant Sci 13:97–119
Justesen AF, Ridoutb CJ, Hovmøller MS (2002) The recent history of Puccinia striiformis f. sp. tritici in Denmark as revealed by disease incidence and AFLP markers. Plant Pathol 51:13–23
Kolmer JA (1993) Selection in a heterogeneous population of Puccinia recondite f. sp. tritici. Phytopathology 83:909–914
Kolmer JA, Liu JQ, Sies M (1995) Virulence and molecular polymorphism in Puccinia recondita f. sp. tritici in Canada. Phytopathology 85:276–285
Leung H, Nelson RJ, Leach JE (1993) Population structure of plant pathogenic fungi and bacteria. Adv Plant Pathol 10:157–205
Levy M, Correa-Victoria FJ, Zeigler RS, Xu S, Hamer JE (1993) Genetic diversity of the rice blast fungus in a disease nursery in Columbia. Phytopathology 83:1427–1433
Limpert E (1987) Barley mildew in Europe: evidence of wind dispersal of the pathogen and its implications for improved use of host resistance and of fungicides for mildew control. In: Wolfe MS, Limpert E (eds) Integrated control of cereal mildews: monitoring the pathogen. Martinus Nijhoff, Dordrecht, pp 31–34
Limpert E, Clifford B, Dreiseitl A, Johnson R, Müller K, Roelfs A, Wellings C (1994) Systems of designation of pathotypes of plant pathogens. J Phytopathol 140:359–362
Majer D, Mithen R, Lewis BG, Vos P, Oliver RP (1996) The use of AFLP fingerprinting for the detection of genetic variation in fungi. Mycol Res 9:1107–1111
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
McDonald BA, McDermott JM (1993) Population genetics of plant pathogenic fungi. Bioscience 43:311–319
Müller K, McDermott JM, Wolfe MS, Limpert E (1996) Analysis of diversity of plant pathogens: the barley powdery mildew pathogen across Europe. Eur J of Plant Pathol 102:385–395
Nelson RJ, Baraoidan MR, Vera Cruz CM, Yap IV, Leach JE, Mew TW, Leung H (1994) Relationship between phylogeny and pathotype for the bacterial blight pathogen of rice. Appl Environ Microb 60:3275–3283
Newton AC, Hackett CA, Guy DC (1998) Diversity and complexity of Erysiphe graminis f. sp. hordei collected from barley cultivar mixtures or barley plots treated with a resistance elicitor. Eur J Plant Pathol 104:925–931
Parks R, Carbone I, Murphy JP, Cowger C (2009) Population genetic analysis of an eastern U.S. wheat powdery mildew population reveals geographic subdivision and recent common ancestry with U.K. and Israeli populations. Phytopathology 99:840–849
Paul S, Wachira FN, Powell W, Waugh R (1997) Diversity and genetic differentiation among populations of Indian and Kenyan tea (Camellia sinensis (L.) O. Kuntze) revealed by AFLP markers. Theor Appl Genet 94:255–263
Péros JP, Troulet C, Guerriero M, Michel-Romiti C, Notteghem JL (2005) Genetic variation and population structure of the grape powdery mildew fungus, Erysiphe necator, in southern France. Eur J of Plant Pathol 113:407–416
Ploetz RC, Schnell RJ, Ying Z, Zheng Q, Olano CT, Motamayor JC, Johnson ES (2005) Analysis of molecular diversity in Crinipellis perniciosa with AFLP markers. Eur J of Plant Pathol 111:317–326
Rosenberg NA, Nordborg M (2002) Genealogical trees, coalescent theory and the analysis of genetic polymorphisms. Nat Rev Genet 3:380–390
Thuan NTN, Bigirimana J, Roumen E, van der Straeten D, Höftel M (2006) Molecular and pathotype analysis of the rice blast fungus in North Vietnam. Eur J Plant Pathol 114:381–396
Tooley PW, O’Neill NR, Goley ED, Carras MM (2000) Assessment of diversity in Claviceps africana and other Claviceps species by RAM and AFLP analyses. Phytopathology 90:1126–1130
Tucker MA, Moffat CS, Ellwood SR, Tan KC, Jayasena K, Oliver RP (2015) Development of genetic SSR markers in Blumeria graminis f. sp. hordei and application to isolates from Australia. Plant Pathol 64:337–343
van de Peer Y, de Wachter R (1994) TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput Appl Biosci 10:569–570
Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Wang M, Xue F, Yang P, Duan X, Zhou Y, Shen C, Zhang G, Wang B (2014) Development of SSR markers for a phytopathogenic fungus, Blumeria graminis f.sp. tritici, using a FIASCO Protocol. J Integr Agr 13:100–104
Whitlock MC, McCauley DE (1999) Indirect measures of gene flow and migration: F ST ≠ 1/(4Nm + 1). Heredity 82:117–125
Wolfe MS, McDermott JM (1994) Population genetics of plant pathogen interactions: the example of the Erysiphe graminis–Hordeum vulgare pathosystem. Annu Rev Phytopathol 32:89–113
You Q (2001) Discuss the differences of wind between winter and summer in Yunnan. Geogr Educ 2:40
Yu D, Le-Sang GD, Ou Z, Ma D (1998) A review on study of wild barley germplasm resources in Tibet. Tibet J Agric Sci 20:14–19
Zabeau M, Vos P (1993) Selective restriction fragment amplification, a method for DNA finger-printing. Publication 0 534 858 AI Bulletin 93/13, European Patent Office, Paris
Zhang Z, Henderson C, Perfect E, Carver TLW, Thomas BJ, Skamnioti P, Gurr SJ (2005) Of genes and genomes, needles and haystacks: Blumeria graminis and functionality. Mol Plant Pathol 6:561–575
Zheng J, Yang Z, Qu S (2013) Progress on barley production and its resistance to powdery mildew in Yunnan province. Barley Cereal Sci 1:36–39
Zhong S, Steffenson BJ (2000) A simple and sensitive silver staining method for detecting AFLP markers in fungi. Fungal Genet Newslett 47: 101–102. Available via DIALOG. http://www.fgsc.net/fgn47/pdfs/FGN47zhong.pdf. Cited 16 Jan 1999
Zhong S, Steffenson BJ (2001) Virulence and molecular diversity in Cochliobolus sativus. Phytopathology 91:469–476
Zhu J, Wang J, Jia Q, Lin F, Zhou Y, Yang J (2009) Investigation on virulence of Blumeria graminis f. sp. hordei population and resistance to powdery mildew in barley varieties (lines). Acta Phytophy Sinica 6:509–516
Zhu J, Wang J, Jia Q, Yang J, Zhou Y, Lin F, Hua W, Shang Y (2010) Pathotypes and genetic diversity of Blumeria graminis f. sp. hordei in the winter barley regions in China. Agr Sci China 9:1787–1798
Acknowledgments
We thank Professor He Chen, Yancheng Academy of Agricultural Sciences, Yancheng, China, Associate Professor Shujie Wang, Zhumadian Institute of Agricultural Sciences, Zhumadian, China, Professor Delu Chen, Putian Institute of Agricultural Sciences, Putian, China, and Professor Zaixin Li, Jingzhou Academy of Agricultural Sciences, Jingzhou, China, for collecting Bgh isolates. We thank Professor Xianyu Duan, Institution of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing, China, for revising the paper carefully. This work was financially supported by the Special Fund for Agro-scientific Research in the Public Interest (201303016), the Public Benefit Technology Applied Research Project of Zhejiang Province (2014C32016), the China Agriculture Research System (CARS-05) and the National Natural Science Foundation of China (30671289, 31101149).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, J., Zhou, Y., Shang, Y. et al. Genetic evidence of local adaption and long distance migration in Blumeria graminis f. sp. hordei populations from China. J Gen Plant Pathol 82, 69–81 (2016). https://doi.org/10.1007/s10327-016-0643-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-016-0643-1