Abstract
Pyricularia oryzae (Magnaporthe oryzae), a causal agent of blast diseases on staple gramineous crops, is a model organism listed as the most important economically and scientifically of the top 10 fungal pathogens by fungal molecular pathologists. Although we are now in an era of genome-enabled analysis, we need to understand the history of the pathogen’s taxonomy, classification, and parasitic specialization in addition to recent research advances. In this review, we focus on these rather fundamental topics. First, the history of classification, including the discovery of its sexual stage and designation, is overviewed. Based on recent results of phylogenetic analysis of Magnaporthaceae isolates, blast fungi are suggested to constitute a robust population that is not congeneric with Magnaporthe salvinii, the type species of Magnaporthe. Second, genetic mechanisms involved in its parasitic specialization into host-specific subgroups and races are outlined. Implications of recent molecular data for resistance breeding are discussed.




Similar content being viewed by others
References
Barr ME (1977) Magnaporthe, Telimenella, and Hyponectria (Physosporellaceae). Mycologia 69:952–966
Böhnert HU, Fudal I, Dioh W, Tharreau D, Notteghem J-L, Lebrun M-H (2004) A putative polyketide synthase/peptide synthetase from Magnaporthe grisea signals pathogen attack to resistant rice. Plant Cell 16:2499–2513
Chuma I, Isobe C, Hotta Y, Ibaragi K, Futamata N, Kusaba M, Yoshida K, Terauchi R, Fujita Y, Nakayashiki H, Valent B, Tosa Y (2011) Multiple translocation of the AVR-Pita effector gene among chromosomes of the rice blast fungus Magnaporthe oryzae and related species. PLoS Pathogens :e1002147
Couch BC, Kohn LM (2002) A multilocus gene genealogy concordant with host preference indicates segregation of a new species, Magnaporthe oryzae, from M. grisea. Mycologia 94:683–693
Couch BC, Fudal I, Lebrun M-H, Tharreau D, Valent B, van Kim P, Nottéghem J-L, Kohn LM (2005) Origins of host-specific populations of the blast pathogen Magnaporthe oryzae in crop domestication with subsequent expansion of pandemic clones on rice and weeds of rice. Genetics 170:613–630
Dai Y, Jia Y, Correll J, Wang X, Wang Y (2010) Diversification and evolution of the avirulence gene AVR-Pita1 in field isolates of Magnaporthe oryzae. Fungal Genet Biol 47:973–980
Dean RA, Talbot NJ, Ebbole DJ, Farman ML, Mitchell TK, Orbach MJ, Thon M, Kulkarni R, Xu J-R, Pan H, Read ND, Lee Y-H, Carbone I, Brown D, Oh YY, Donofrio N, Jeong JS, Soanes DM, Djonovic S, Kolomiets E, Rehmeyer C, Li W, Harding M, Kim S, Lebrun M-H, Bohnert H, Coughlan S, Butler J, Calvo S, Ma L-J, Nicol R, Purcell S, Nusbaum C, Galagan JE, Birren BW (2005) The genome sequence of the rice blast fungus Magnaporthe grisea. Nature 434:980–986
Dean R, Van Kan JAL, Pretorius ZA, Hammond-Kosack KE, Pietro AD, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J, Foster GD (2012) The top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol 13:414–430
Eto Y, Ikeda K, Chuma I, Kataoka T, Kuroda S, Kikuchi N, Don LD, Kusaba M, Nakayashiki H, Tosa Y, Mayama S (2001) Comparative analyses of the distribution of various transposable elements in Pyricularia and their activity during and after the sexual cycle. Mol Gen Genet 264:565–577
Farman ML (2002) Pyricularia grisea isolates causing gray leaf spot on perennial ryegrass (Lolium perenne) in the United States: relationship to P. grisea isolates from other host plants. Phytopathology 92:245–254
Fetch T Jr, Johnston PA, Pickering R (2009) Chromosomal location and inheritance of stem rust resistance transferred from Hordeum bulbosum into cultivated barley (H. vulgare). Phytopathology 99:339–343
Hawksworth DL (2011) A new dawn for the naming of fungi: impacts of decisions made in Melbourne in July 2011 on the future publication and regulation of fungal names. IMA Fungus 2:155–162
Hebert TT (1971) The perfect stage of Pyricularia grisea. Phytopathology 61:83–87
Hirata K, Kusaba M, Chuma I, Osue J, Nakayashiki H, Mayama S, Tosa Y (2007) Speciation in Pyricularia inferred from multilocus phylogenetic analysis. Mycol Res 111:799–808
Kang S, Sweigard JA, Valent B (1995) The PWL host specificity gene family in the blast fungus Magnaporthe grisea. Mol Plant Microb Interact 8:939–948
Kang S, Lebrun MH, Farrall L, Valent B (2001) Gain of virulence caused by insertion of a Pot3 transposon in a Magnaporthe grisea avirulence gene. Mol Plant Microb Interact 14:671–674
Kato H (1978) Biological and genetic aspects in the perfect state of rice blast fungus, Pyricularia oryzae Cav. and its allies. Gamma Field Symposia 17:1–22
Kato H, Yamaguchi T, Nishihara N (1976) The perfect state of Pyricularia oryzae Cav. in culture. Ann Phytopath Soc Jpn 42:507–510
Kato H, Yamamoto M, Yamaguchi-Ozaki T, Kadouchi H, Iwamoto Y, Nakayashiki H, Tosa Y, Mayama S, Mori N (2000) Pathogenicity, mating ability and DNA restriction fragment length polymorphisms of Pyricularia populations isolated from Gramineae, Bambusideae and Zingiberaceae plants. J Gen Plant Pathol 66:30–47
Kiyosawa S (1982) Genetics and epidemiological modeling of breakdown of plant disease resistance. Ann Rev Phytopathol 20:93–117
Landschoot PJ, Hoyland BF (1992) Gray leaf spot of perennial ryegrass turf in Pennsylvania. Plant Dis 76:1280–1282
Li W, Wang B, Wu J, Lu G, Hu Y, Zhang X, Zhang Z, Zhao Q, Feng Q, Zhang H, Wang Z, Wang G, Han B, Wang Z, Zhou B (2009) The Magnaporthe oryzae avirulence gene AvrPiz-t encodes a predicted secreted protein that triggers the immunity in rice mediated by the blast resistance gene Piz-t. Mol Plant Microb Interact 22:411–420
Luo J, Zhang N (2013) Magnaporthiopsis, a new genus in Magnaporthaceae (Ascomycota). Mycologia 105:1019–1029
Miki S, Matsui K, Kito H, Otsuka K, Ashizawa T, Yasuda N, Fukiya S, Sato J, Hirayae K, Fujita Y, Nakajima T, Tomita F, Sone T (2009) Molecular cloning and characterization of the AVR-Pia locus from a Japanese field isolate of Magnaporthe oryzae. Mol Plant Pathol 10:361–374
Murakami J, Tosa Y, Kataoka T, Tomita R, Kawasaki J, Chuma I, Sesumi Y, Kusaba M, Nakayashiki H, Mayama S (2000) Analysis of host species specificity of Magnaporthe grisea toward wheat using a genetic cross between isolates from wheat and foxtail millet. Phytopathology 90:1060–1067
Murata N, Aoki T, Kusaba M, Tosa Y, Chuma I (2014) Various species of Pyricularia constitute a robust clade distinct from Magnaporthe salvinii and its relatives in Magnaporthaceae. J Gen Plant Pathol 80:66–72
Oh HS, Tosa Y, Takabayashi N, Nakagawa S, Tomita R, Don LD, Kusaba M, Nakayashiki H, Mayama S (2002) Characterization of an Avena isolate of Magnaporthe grisea and identification of a locus conditioning its specificity on oat. Can J Bot 80:1088–1095
Orbach MJ, Farrall L, Sweigard JA, Chumley FG, Valent B (2000) A telomeric avirulence gene determines efficacy for the rice blast resistance gene Pi-ta. Plant Cell 12:2019–2032
Pratt K (2012) UK researchers find important new disease. UKAgNews, 24 April 2012. University of Kentucky, College of Agriculture, Food and Environment, Lexington, KY. http://news.ca.uky.edu/article/uk-researchers-find-important-new-disease. Accessed 2 Mar 2014
Rossman AY, Howard RJ, Valent B (1990) Pyricularia grisea, the correct name for the rice blast disease fungus. Mycologia 82:509–512
Sanchez E Jr, Asano K, Sone T (2011) Characterization of Inago1 and Inago2 retrotransposons in Magnaporthe oryzae. J Gen Plant Pathol 77:239–242
Silué D, Notteghem JL, Tharreau D (1992) Evidence of a gene-for-gene relationship in the Oryza sativa–Magnaporthe grisea pathosystem. Phytopathology 82:577–580
Sweigard JA, Carroll AM, Kang S, Farrall L, Chumley FG, Valent B (1995) Identification, cloning, and characterization of PWL2, a gene for host species specificity in the rice blast fungus. Plant Cell 7:1221–1233
Takabayashi N, Tosa Y, Oh HS, Mayama S (2002) A gene-for-gene relationship underlying the species-specific parasitism of Avena/Triticum isolates of Magnaporthe grisea on wheat cultivars. Phytopathology 92:1182–1188
Takahashi M, Ashizawa T, Hirayae K, Moriwaki J, Sone T, Sonoda R, Noguchi MT, Nagashima S, Ishikawa K, Arai M (2010) One of two major paralogs of AVR-Pita1 is functional in Japanese rice blast isolates. Phytopathology 100:612–618
Tosa Y, Chuma I (2011) Genetic analyses of “host species specificity” of Magnaporthe oryzae/grisea. In: Wolpert T, Shiraishi T, Collmer A, Akimitsu K, Glazebrook J (eds) Genome-enabled analysis of plant-pathogen interactions. APS Press, St. Paul, pp 93–99
Tosa Y, Nakayashiki H, Hyodo H, Mayama S, Kato H, Leong SA (1995) Distribution of retrotransposon MAGGY in Pyricularia species. Ann Phytopathol Soc Jpn 61:549–554
Tosa Y, Hirata K, Tamba H, Nakagawa S, Chuma I, Isobe C, Osue J, Urashima AS, Don LD, Kusaba M, Nakayashiki H, Tanaka A, Tani T, Mori N, Mayama S (2004) Genetic constitution and pathogenicity of Lolium isolates of Magnaporthe oryzae in comparison with host species-specific pathotypes of the blast fungus. Phytopathology 94:454–462
Tosa Y, Tamba H, Tanaka K, Mayama S (2006) Genetic analysis of host species specificity of Magnaporthe oryzae isolates from rice and wheat. Phytopathology 96:480–484
Tsuda M, Ueyama A (1982) A comment from a taxonomical viewpoint on the perfect state of the blast fungus (abstract in Japanese). Ann Phytopath Soc Japan 48:340
Ueyama A, Tsuda M (1975) Formation of the perfect state in culture of Pyricularia sp. from some graminaceous plants (preliminary report). Trans Mycol Soc Jpn 16:420–422
Urashima AS, Igarashi S, Kato H (1993) Host range, mating type, and fertility of Pyricularia grisea from wheat in Brazil. Plant Dis 77:1211–1216
Valent B, Chumley FG (1991) Molecular genetic analysis of the rice blast fungus, Magnaporthe grisea. Ann Rev Phytopathol 29:443–467
Wingfield MJ, De Beer ZW, Slippers B, Wingfield BD, Groenewald JZ, Lombard L, Crous PW (2012) One fungus, one name promotes progressive plant pathology. Mol Plant Pathol 13:604–613
Yaegashi H (1978) Inheritance of pathogenicity in crosses of Pyricularia isolates from weeping lovegrass and finger millet. Ann Phytopath Soc Jpn 44:626–632
Yaegashi H (1981) Studies on the perfect stage of Pyricularia species (in Japanese). Bull Tohoku Natl Agric Exp Stn 63:49–125
Yaegashi H, Nishihara N (1976) Production of the perfect stage in Pyricularia from cereals and grasses. Ann Phytopath Soc Japan 42:511–515
Yaegashi H, Udagawa S (1978) The taxonomical identity of the perfect state of Pyricularia grisea and its allies. Can J Bot 56:180–183
Yoshida K, Saitoh H, Fujisawa S, Kanzaki H, Matsumura H, Yoshida K, Tosa Y, Chuma I, Takano Y, Win J, Kamoun S, Terauchi R (2009) Association genetics reveals three novel avirulence genes from the rice blast fungal pathogen Magnaporthe oryzae. Plant Cell 21:1573–1591
Zhang N, Zhao S, Shen Q (2011) A six-gene phylogeny reveals the evolution of mode of infection in the rice blast fungus and allied species. Mycologia 103:1267–1276
Zhou E, Jia Y, Singh P, Correll JC, Lee FN (2007) Instability of the Magnaporthe oryzae avirulence gene AVR-Pita alters virulence. Fungal Genet Biol 44:1024–1034
Acknowledgments
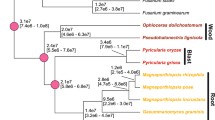
We thank Dr. T. Aoki, National Institute of Agrobiological Sciences, Japan, for valuable comments on fungal taxonomy and Dr. M. Kusaba, Saga University, and Mr. N. Murata, Kobe University, for useful suggestions on phylogenetic analyses, and Dr. Y. Inoue, Kobe University, for drawing Fig. 2.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tosa, Y., Chuma, I. Classification and parasitic specialization of blast fungi. J Gen Plant Pathol 80, 202–209 (2014). https://doi.org/10.1007/s10327-014-0513-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10327-014-0513-7