Abstract
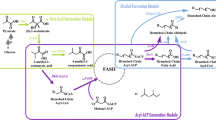
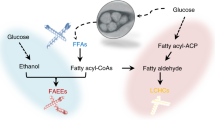
The recently engineered reversal of the β-oxidation cycle has been proposed as a potential platform for the efficient synthesis of longer chain (C ≥ 4) fuels and chemicals. Here, we demonstrate the utility of this platform for the synthesis of medium-chain length (C6–C10) products through the manipulation of key components of the pathway. Deletion of endogenous thioesterases provided a clean background in which the expression of various thiolase and termination components, along with required core enzymes, resulted in the ability to alter the chain length distribution and functionality of target products. This approach enabled the synthesis of medium-chain length carboxylic acids and primary alcohols from glycerol, a low-value feedstock. The use of BktB as the thiolase component with thioesterase TesA’ as the termination enzyme enabled the synthesis of about 1.3 g/L C6–C10 saturated carboxylic acids. Tailoring of product formation to primary alcohol synthesis was achieved with the use of various acyl-CoA reductases. The combination of AtoB and FadA as the thiolase components with the alcohol-forming acyl-CoA reductase Maqu2507 from M. aquaeolei resulted in the synthesis of nearly 0.3 g/L C6–C10 alcohols. These results further demonstrate the versatile nature of a β-oxidation reversal, and highlight several key aspects and control points that can be further manipulated to fine-tune the synthesis of various fuels and chemicals.






Similar content being viewed by others
References
Buchholz K, Collins J (2013) The roots-a short history of industrial microbiology and biotechnology. Appl Microbiol Biotechnol 97:3747–3762
Cho HS, Cronan JE (1993) Escherichia coli thioesterase I, molecular cloning and sequencing of the structural gene and identification as a periplasmic enzyme. J Biol Chem 268:9238–9245
Cintolesi A, Clomburg JM, Gonzalez R (2014) In silico assessment of the metabolic capabilities of an engineered functional reversal of the β-oxidation cycle for the synthesis of longer-chain (C ≥ 4) products. Metab Eng 23:100–115
Clomburg JM, Blankschien MD, Vick JE, Chou A, Kim S, Gonzalez R (2014) Integrated engineering of β-oxidation reversal and ω-oxidation pathways for the synthesis of medium chain ω-functionalized carboxylic acids. Metab Eng. doi:10.1016/j.ymben.2015.01.007
Clomburg JM, Vick JE, Blankschien MD, Rodriguez-Moya M, Gonzalez R (2012) A synthetic biology approach to engineer a functional reversal of the β-oxidation cycle. ACS Synth Biol 1:541–554
Dellomonaco C, Clomburg JM, Miller EN, Gonzalez R (2011) Engineered reversal of the β-oxidation cycle for the synthesis of fuels and chemicals. Nature 476:355–359
Feng Y, Cronan JE (2009) A new member of the Escherichia coli fad regulon: transcriptional regulation of fadM (ybaW). J Bacteriol 191:6320–6328
Fischer B, Boutserin S, Mazon H, Collin S, Branlant G, Gruez A et al (2013) Catalytic properties of a bacterial acylating acetaldehyde dehydrogenase: evidence for several active oligomeric states and coenzyme a activation upon binding. Chem-Biol Interact 202:70–77
Fontaine L, Meynial-Salles I, Girbal L, Yang XH, Croux C, Soucaille P (2002) Molecular characterization and transcriptional analysis of adhE2, the gene encoding the NADH-dependent aldehyde/alcohol dehydrogenase responsible for butanol production in alcohologenic cultures of Clostridium acetobutylicum ATCC 824. J Bacteriol 184:821–830
Gulevich AY, Skorokhodova AY, Sukhozhenko AV, Shakulov RS, Debabov VG (2012) Metabolic engineering of Escherichia coli for 1-butanol biosynthesis through the inverted aerobic fatty acid beta-oxidation pathway. Biotechnol Lett 34:463–469
Gully D, Bouveret E (2006) A protein network for phospholipid synthesis uncovered by a variant of the tandem affinity purification method in Escherichia coli. Proteomics 6:282–293
Hofvander P, Doan TTP, Hamberg M (2011) A prokaryotic acyl-CoA reductase performing reduction of fatty acyl-CoA to fatty alcohol. FEBS Lett 585:3538–3543
Ishige T, Tani A, Takabe K, Kawasaki K, Sakai Y, Kato N (2002) Wax ester production from n-alkanes by Acinetobacter sp strain M-1: Ultrastructure of cellular inclusions and role of acyl coenzyme A reductase. Appl Environ Microbiol 68:1192–1195
Jang YS, Kim B, Shin JH, Choi YJ, Choi S, Song CW et al (2012) Bio-based production of C2–C6 platform chemicals. Biotechnol Bioeng 109:2437–2459
Kang YS, Durfee T, Glasner JD, Qiu Y, Frisch D, Winterberg KM et al (2004) Systematic mutagenesis of the Escherichia coli genome. J Bacteriol 186:4921–4930
Latham JA, Chen DQ, Allen KN, Dunaway-Mariano D (2014) Divergence of substrate specificity and function in the Escherichia coli hotdog-fold thioesterase paralogs YdiI and YbdB. Biochemistry 53:4775–4787
Lenneman EM, Ohlert JM, Palani NP, Barney BM (2013) Fatty alcohols for wax esters in Marinobacter aquaeolei VT8: two optional routes in the wax biosynthesis pathway. Appl Environ Microbiol 79:7055–7062
Lian J, Zhao H (2014) Reversal of the β-oxidation cycle in Saccharomyces cerevisiae for production of fuels and chemicals. ACS Synth Biol. doi:10.1021/sb500243c
Liu R, Zhu FY, Lu L, Fu AS, Lu JK, Deng ZX et al (2014) Metabolic engineering of fatty acyl-ACP reductase-dependent pathway to improve fatty alcohol production in Escherichia coil. Metab Eng 22:10–21
Machado HB, Dekishima Y, Luo H, Lan EI, Liao JC (2012) A selection platform for carbon chain elongation using the CoA-dependent pathway to produce linear higher alcohols. Metab Eng 14:504–511
Martin CH, Dhamankar H, Tseng HC, Sheppard MJ, Reisch CR, Prather KLJ (2013) A platform pathway for production of 3-hydroxyacids provides a biosynthetic route to 3-hydroxy-gamma-butyrolactone. Nat Commun 4:9
Mazumdar S, Blankschien MD, Clomburg JM, Gonzalez R (2013) Efficient synthesis of L-lactic acid from glycerol by metabolically engineered Escherichia coli. Microb Cell Fact 12:7–11
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, New York
Neidhardt FC, Bloch PL, Smith DF (1974) Culture Medium for Enterobacteria. J Bacteriol 119:736–747
Nie L, Ren Y, Janakiraman A, Smith S, Schulz H (2008) A novel paradigm of fatty acid β-oxidation exemplified by the thioesterase-dependent partial degradation of conjugated linoleic acid that fully supports growth of Escherichia coli. Biochemistry 47:9618–9626
Pick A, Ruhmann B, Schmid J, Sieber V (2013) Novel CAD-like enzymes from Escherichia coli K-12 as additional tools in chemical production. Appl Microbiol Biotechnol 97:5815–5824
Reiser S, Somerville C (1997) Isolation of mutants of Acinetobacter calcoaceticus deficient in wax ester synthesis and complementation of one mutation with a gene encoding a fatty acyl coenzyme a reductase. J Bacteriol 179:2969–2975
Riley M, Abe T, Arnaud MB, Berlyn MKB, Blattner FR, Chaudhuri RR et al (2006) Escherichia coli K-12: a cooperatively developed annotation snapshot-2005. Nucleic Acids Res 34:1–9
Rodriguez GM, Atsumi S (2014) Toward aldehyde and alkane production by removing aldehyde reductase activity in Escherichia coli. Metab Eng 25:227–237
Sambrook J, Fritsch EF, Maniatis T, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Schirmer A, Rude MA, Li XZ, Popova E, del Cardayre SB (2010) Microbial Biosynthesis of Alkanes. Science 329:559–562
Straathof AJJ (2014) Transformation of biomass into commodity chemicals using enzymes or cells. Chem Rev 114:1871–1908
Toth J, Ismaiel AA, Chen JS (1999) The ald gene, encoding a coenzyme A-acylating aldehyde dehydrogenase, distinguishes Clostridium beijerinckii and two other solvent-producing clostridia from Clostridium acetobutylicum. Appl Environ Microbiol 65:4973–4980
Vick JE, Clomburg JM, Blankschien MD, Chou A, Kim S, Gonzalez R (2015) FabI and other bacterial enoyl-acyl carrier protein reductases (ENR) support efficient operation of a functional reversal of the β-oxidation cycle. Appl Environ Microbiol 81 doi:10.1128/AEM.03521-14
Willis RM, Wahlen BD, Seefeldt LC, Barney BM (2011) Characterization of a fatty acyl-CoA reductase from Marinobacter aquaeolei VT8: a bacterial enzyme catalyzing the reduction of fatty acyl-CoA to fatty alcohol. Biochemistry 50:10550–10558
Zhu HL, Gonzalez R, Bobik TA (2011) Coproduction of acetaldehyde and hydrogen during glucose fermentation by Escherichia coli. Appl Environ Microbiol 77:6441–6450
Zhuang Q, Wang Q, Liang Q, Qi Q (2014) Synthesis of polyhydroxyalkanoates from glucose that contain medium-chain-length monomers via the reversed fatty acid β-oxidation cycle in Escherichia coli. Metab Eng 24:78–86
Zhuang ZH, Song F, Zhao H, Li L, Cao J, Eisenstein E et al (2008) Divergence of function in the hot dog fold enzyme superfamily: the bacterial thioesterase YciA. Biochemistry 47:2789–2796
Acknowledgments
This work was supported by grants from the U.S. National Science Foundation (CBET-1134541, CBET-1067565).
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: Metabolic Engineering.
Rights and permissions
About this article
Cite this article
Kim, S., Clomburg, J.M. & Gonzalez, R. Synthesis of medium-chain length (C6–C10) fuels and chemicals via β-oxidation reversal in Escherichia coli . J Ind Microbiol Biotechnol 42, 465–475 (2015). https://doi.org/10.1007/s10295-015-1589-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-015-1589-6