Abstract
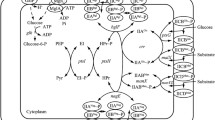
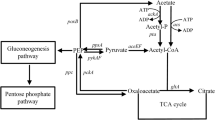
High concentrations of acetate, the main by-product of Escherichia coli (E. coli) high cell density culture, inhibit bacterial growth and l-threonine production. Since metabolic overflux causes acetate accumulation, we attempted to reduce acetate production by redirecting glycolysis flux to the pentose phosphate pathway by deleting the genes encoding phosphofructokinase (pfk) and/or pyruvate kinase (pyk) in an l-threonine-producing strain of E. coli, THRD. pykF, pykA, pfkA, and pfkB deletion mutants produced less acetate (9.44 ± 0.83, 3.86 ± 0.88, 0.30 ± 0.25, and 6.99 ± 0.85 g/l, respectively) than wild-type THRD cultures (19.75 ± 0.93 g/l). THRDΔpykF and THRDΔpykA produced 11.05 and 5.35 % more l-threonine, and achieved a 10.91 and 5.60 % higher yield on glucose, respectively. While THRDΔpfkA grew more slowly and produced less l-threonine than THRD, THRDΔpfkB produced levels of l-threonine (102.28 ± 2.80 g/l) and a yield on glucose (0.34 g/g) similar to that of THRD. The dual deletion mutant THRDΔpfkBΔpykF also achieved low acetate (7.42 ± 0.81 g/l) and high l-threonine yields (111.37 ± 2.71 g/l). The level of NADPH in THRDΔpfkA cultures was depressed, whereas all other mutants produced more NADPH than THRD did. These results demonstrated that modification of glycolysis in E. coli THRD reduced acetate production and increased accumulation of l-threonine.




Similar content being viewed by others
References
Aristidou AA, San KY, Bennett GN (1999) Improvement of biomass yield and recombinant gene expression in Escherichia coli by using fructose as the primary carbon source. Biotechnol Prog 15(1):140–145. doi:10.1021/bp980115v
Ausubel FM, Brent R, Kington RE, Seidman JG, Smith JA, Struhl K (1992) Short protocols in molecular biology, 3rd edn. Wiley, New York
Babu KR, Swaminathan S, Marten S, Khanna N, Rinas U (2000) Production of interferon-α in high cell density cultures of recombinant Escherichia coli and its single step purification from refolded inclusion body proteins. Appl Microbiol Biotechnol 53(6):655–660. doi:10.1007/s002530000318
Blombach B, Seibold GM (2010) Carbohydrate metabolism in Corynebacterium glutamicum and applications for the metabolic engineering of l-lysine production strains. Appl Microbiol Biotechnol 86(5):1313–1322. doi:10.1007/s00253-010-2537-z
Chang DE, Shin S, Rhee JS, Pan JG (1999) Acetate metabolism in a pta mutant of Escherichia coli W3110: importance of maintaining acetyl-coenzyme A flux for the growth and survival. J Bacteriol 181(21):6656–6663
Chin JW, Cirino PC (2011) Improved NADPH supply for xylitol production by engineered Escherichia coli with glycolytic mutations. Biotechnol Prog 27(2):333–341. doi:10.1002/btpr.559
Choi JH, Keum KC, Lee SY (2006) Production of recombinant proteins by high cell density culture of Escherichia coli. Chem Eng Sci 61(3):876–885. doi:10.1016/j.ces.2005.03.031
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97(12):6640–6645. doi:10.1073/pnas.120163297
De Mey M, De Maeseneire S, Soetaert W, Vandamme E (2007) Minimizing acetate formation in E. coli fermentations. J Ind Microbiol Biotechnol 34(11):689–700. doi:10.1007/s10295-007-0244-2
Dong XY, Quinn PJ, Wang XY (2011) Metabolic engineering of Escherichia coli and Corynebacterium glutamicum for the production of l-threonine. Biotechnol Adv 29(1):11–23. doi:10.1016/j.biotechadv.2010.07.009
Eiteman MA, Altman E (2008) Overcoming acetate in Escherichia coli recombinant protein fermentations. Trends Biotechnol 24(11):530–536. doi:10.1016/j.tibtech.2006.09.001
Fry B, Zhu T, Domach MM, Koepsel RR, Phalakornkule C, Ataai MM (2000) Characterization of growth and acid formation in a Bacillus subtilis pyruvate kinase mutant. Appl Environ Microbiol 66(9):4045–4049. doi:10.1128/AEM.66.9.4045-4049.2000
Hoque MA, Ushiyama H, Tomita M, Shimizu K (2005) Dynamic responses of the intracellular metabolite concentrations of the wild type and pykA mutant Escherichia coli against pulse addition of glucose or NH3 under those limiting continuous cultures. Biochem Eng J 26(1):38–49. doi:10.1016/j.bej.2005.05.012
Johnston W, Cord-Ruwisch R, Cooney M (2002) Industrial control of recombinant Escherichia coli fed-batch culture: new perspectives on traditional controlled variables. Bioprocess Biosyst Eng 25(2):111–120. doi:10.1007/s00449-002-0287-8
Kedar P, Colah R, Shimizu K (2007) Proteomic investigation on the pyk-F gene knockout Escherichia coli for aromatic amino acid production. Enzym Microb Tech 41(4):455–465. doi:10.1016/j.enzmictec.2007.03.018
Korz DJ, Rinas U, Hellmuth K, Sanders EA, Deckwer WD (1995) Simple fed-batch technique for high cell density cultivation of Escherichia coli. J Biotechnol 39(1):59–65. doi:10.1016/0168-1656(94)00143-Z
Lara AR, Vazquez-Limon C, Gosset G, Bolivar F, Lopez-Munguia A, Ramirez OT (2006) Engineering Escherichia coli to improve culture performance and reduce formation of by-products during recombinant protein production under transient intermittent anaerobic conditions. Biotechnol Bioeng 94(6):164–175. doi:10.1002/bit.20954
Lee SY (1996) High cell-density culture of Escherichia coli. Trends Biotechnol 14(3):98–105. doi:10.1016/0167-7799(96)80930-9
Lovingshimer MR, Siegele D, Reinhart GD (2006) Construction of an inducible, pfkA and pfkB deficient strain of Escherichia coli for the expression and purification of phosphofructokinase from bacterial sources. Protein Expr Purif 46(2):475–482. doi:10.1016/j.pep.2005.09.015
Marx A, Hans S, Möckel B, Bathe B, de Graaf AA, McCormack AC, Stapleton C, Burke K, O’Donohue M, Dunican LK (2003) Metabolic phenotype of phosphoglucose isomerase mutants of Corynebacterium glutamicum. J Biotechnol 104(1–3):185–197. doi:10.1016/S0168-1656(03)00153-6
Muñoz-Márquez ME, Ponce-Rivas E (2010) Effect of pfkA chromosomal interruption on growth, sporulation, and production of organic acids in Bacillus subtilis. J Basic Microbiol 50(3):232–240. doi:10.1002/jobm.200900236
Park JH, Lee KH, Kim TY, Lee SY (2007) Metabolic engineering of Escherichia coli for the production of l-valine based on transcriptome analysis and in silico gene knockout simulation. Proc Natl Acad Sci U S A 104(19):7797–7802. doi:10.1073/pnas.0702609104
Picon A, Teixeira de Mattos MJ, Postma PW (2005) Reducing the glucose uptake rate in Escherichia coli affects growth rate but not protein production. Biotechnol Bioeng 90(2):191–200. doi:10.1002/bit.20387
Ponce E (1999) Effect of growth rate reduction and genetic modifications on acetate accumulation and biomass yields in Escherichia coli. J Biosci Bioeng 87(6):775–780. doi:10.1016/S1389-1723(99)80152-2
Rieping M, Hermann T (2006) l-Threonine. In: Wendisch VF (ed) Amino acid biosynthesis – pathways, regulation and metabolic engineering. Springer, Berlin Heidelberg, New York, pp 71–92
Rodríguez-Prados JC, de Atauri P, Maury J, Ortega F, Portais JC, Chassagnole C, Acerenza L, Lindley ND, Cascante M (2009) In silico strategy to rationally engineer metabolite production: a case study for threonine in Escherichia coli. Biotechnol Bioeng 103(3):609–620. doi:10.1002/bit.22271
Siddiquee KA, Arauzo-Bravo MJ, Shimizu K (2004) Effect of a pyruvate kinase (pykF-gene) knockout mutation on the control of gene expression and metabolic fluxes in Escherichia coli. FEMS Microbiol Lett 235(1):25–33. doi:10.1016/j.femsle.2004.04.004
Siddiquee KA, Arauzo-Bravo MJ, Shimizu K (2004) Metabolic flux analysis of pykF gene knockout Escherichia coli based on 13C-labeling experiments together with measurements of enzyme activities and intracellular metabolite concentrations. Appl Microbiol Biotechnol 63(4):407–417. doi:10.1016/j.femsle.2004.04.004
Siedler S, Bringer S, Blank LM, Bott M (2012) Engineering yield and rate of reductive biotransformation in Escherichia coli by partial cyclization of the pentose phosphate pathway and PTS-independent glucose transport. Appl Microbiol Biotechnol 93(4):1459–1467. doi:10.1007/s00253-011-3626-3
Siedler S, Bringer S, Bott M (2011) Increased NADPH availability in Escherichia coli: improvement of the product per glucose ratio in reductive whole-cell biotransformation. Appl Microbiol Biotechnol 92(5):929–937. doi:10.1007/s00253-011-3374-4
Toya Y, Ishii N, Nakahigashi K, Hirasawa T, Soga T, Tomita M, Shimizu K (2010) 13C-metabolic flux analysis for batch culture of Escherichia coli and its pyk and pgi gene knockout mutants based on mass isotopomer distribution of intracellular metabolites. Biotechnol Prog 26(4):975–992. doi:10.1002/btpr.420
Wang Y, San KY, Bennett GN (2013) Improvement of NADPH bioavailability in Escherichia coli through the use of phosphofructokinase deficient strains. Appl Microbiol Biotechnol 97(15):6883–6893. doi:10.1007/s00253-013-4859-0
Zawada J, Swartz J (2005) Maintaining rapid growth in moderate-density Escherichia coli fermentations. Biotechnol Bioeng 89(4):407–415. doi:10.1002/bit.20369
Zerez CR, Moul DE, Gomez EG, Lopez VM, Andreoli AJ (1987) Negative modulation of Escherichia coli NAD kinase by NADPH and NADH. J Bacteriol 169:184–188
Zhu T, Phalakoronkule C, Koepsel RR, Domach MM, Ataai MM (2001) Cell growth and by-product formation in a pyruvate kinase mutant of Escherichia coli. Biotechnol Prog 17(1):624–628. doi:10.1021/bp0100575
Acknowledgments
This research was supported by the National High Technology Research and Development Program (2012AA022102 and 2012AA02A703) and the National Basic Research Program of China (2013CB733901).
Author information
Authors and Affiliations
Corresponding author
Additional information
X. Xie and Y. Liang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xie, X., Liang, Y., Liu, H. et al. Modification of glycolysis and its effect on the production of l-threonine in Escherichia coli . J Ind Microbiol Biotechnol 41, 1007–1015 (2014). https://doi.org/10.1007/s10295-014-1436-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-014-1436-1