Abstract
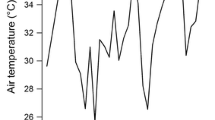
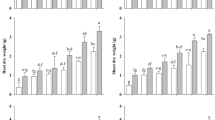
We studied the possible roles of flavonoids in the antioxidant and antiherbivore chemistry in Jatropha curcas (L.), a Latin American shrub that holds great potential as a source of biofuel. Changes in flavonoid concentrations in the leaves of J. curcas seedlings exposed to artificial damage and to different rainfall patterns were assessed by applying a 32-factorial experiment in a greenhouse. The concentrations of different flavonoids in the leaves of seedlings were significantly affected by interaction effects of artificial damage, drought stress and age of the seedling. The highest flavonoid concentrations were obtained in seedlings imposed to the highest percentage of artificial damage (50 %) and grown under extreme drought stress (200 mm year−1). In this treatment combination, flavonoid concentrations were three-fold as compared to seedlings exposed to the same level of artificial damage but grown in 1900 mm year−1 rainfall application. Without artificial damage, the concentration of flavonoids in the seedlings grown in 200 mm year−1 rainfall application was still two-fold compared to seedlings grown in higher (>800 mm year−1) rainfall applications. Thus, the observed flavonoid concentration patterns in the leaves of J. curcas seedlings were primarily triggered by drought stress and light rather than by artificial damage, suggesting that drought causes oxidative stress in J. curcas.



Similar content being viewed by others
References
Abd-Alla HI, Moharram FA, Gaara AH, El-Safty MM (2009) Phytoconstituents of Jatropha curcas L. leaves and their immunomodulatory activity on humoral and cell-mediated immune response in chicks. Z Naturforsch C 64:495–501. doi:10.1515/znc-2009-7-805
Achten WMJ, Trabucco A, Maes WH, Verchot LV, Aerts R, Mathijs E, Vantomme P, Singh VP, Muys B (2013) Global greenhouse gas implications of land conversion to biofuel crop cultivation in arid and semi-arid lands—lessons learned from Jatropha. J Arid Environ 98:135–145. doi:10.1016/j.jaridenv.2012.06.015
Agati G, Tattini M (2010) Multiple functional roles of flavonoids in photo protection. New Phytol 186:786–793. doi:10.1111/j.1469-8137.2010.03269.x
Agati G, Cerovic ZG, Pinelli P, Tattini M (2011) Light-induced accumulation of ortho-dihydroxylated flavonoids as non-destructively monitored by chlorophyll fluorescence excitation techniques. Environ Exp Bot 73:3–9. doi:10.1016/j.envexpbot.2010.10.002
Agati G, Brunetti C, Di Ferdinando M, Ferrini F, Pollastri S, Tattini M (2013) Functional roles of flavonoids in photoprotection: new evidence, lessons from the past. Plant Physiol Biochem 72:35–45. doi:10.1016/j.plaphy.2013.03.014
Agrawal AA (2011) Current trends in the evolutionary ecology of plant defence. Funct Ecol 25:420–432. doi:10.1111/j.1365-2435.2010.01796.x
Alvero-Bascos EM, Ungson LB (2012) Ultraviolet-B (UV-B) radiation as an elicitor of flavonoid production in callus cultures of Jatropha (Jatropha curcas L.). Philipp Agric Sci 95:335–343
Azam MM, Waris A, Nahar NM (2005) Prospects and potential of fatty acid methyl esters of some non-traditional seed oils for use as biodiesel in India. Biomass Bioenerg 29:293–302. doi:10.1016/j.biombioe.2005.05.001
Bandau F, Decker VHG, Gundale MJ, Albrectsen BR (2015) Genotypic tannin levels in Populus tremulaimpact the way nitrogen enrichment affects growth and allocation responses for some traits and not for others. Plus One. doi:10.1371/journal.pone.0140971
Bryant JP, Chapin FS, Klein DR (1983) Carbon nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40:357–368
Close DC, McArthur C (2002) Rethinking the role of many plant phenolics—protection from photo damage not herbivores? Oikos 99:166–172. doi:10.1034/j.1600-0706.2002.990117.x
Coley P, Bryant J, Chapin F III (1985) Resource availability and plant antiherbivore defense. Sciences 230:895–899
Crozier A, Jaganath IB, Clifford MN (2006) Phenols, polyphenols and tannins: an overview. In: Crozier A, Clifford MN, Ashihara H (eds) Plant secondary metabolites: occurrence, structure and role in the human diet. Blackwell Publishing Ltd., Oxford, p 1–24. doi:10.1002/9780470988558.ch1
Cruz de Carvalho MH (2008) Drought stress and reactive oxygen species: production, scavenging and signaling. Plant Signal Behav 3:156–165
Engström MT, Pälijärvi M, Fryganas F, Grabber J, Mueller-Harvey I, Salminen J-P (2014) Rapid qualitative and quantitative analysis of proanthocyanidin oligomers and polymers by UPLC-MS/MS. J Agric Food Chem 62:3390–3399
Engström MT, Pälijärvi M, Salminen J-P (2015) Rapid fingerprint analysis of plant extracts for ellagitannins, gallic acid and quinic acid derivatives, and quercetin-, kaempferol- and myricetin-based flavonol glycosides by UPLC-QqQ-MS/MS. J Agric Food Chem 63:4068–4079
Fairless D (2007) Biofuel: the little shrub that could—maybe. Nature 449:652–655. doi:10.1038/449652a
Feeny P (1976) Plant apparency and chemical defense. Recent Adv Phytochem 10:1–40
Ferdinando M, Brunetti C, Fini A, Tattini M (2012) Flavonoids as antioxidants in plants under abiotic stresses. In: Ahmad P, Prasad MNV (eds) Abiotic stress responsesin plants-metabolism, productivity and sustainability. Springer, New York Dordrecht Heidelberg London, pp 159–179
Fini A, Bellasio C, Pollastri S, Tattini M, Ferrini F (2013) Water relations, growth, and leaf gas exchange as affected by water stress in Jatropha curcas. J Arid Environ 89:21–29. doi:10.1016/j.jaridenv.2012.10.009
Gadd ME, Young TP, Palmer TM (2001) Effects of simulated shoot and leaf herbivory on vegetative growth and plant defense in Acacia drepanolobium. Oikos 92:515–521
Galeotti F, Barile E, Curir P, Dolci M, Lanzotti V (2008) Flavonoids from carnation (Dianthus caryophyllus) and their antifungal activity. Phytochem Lett 1:44–48. doi:10.1016/j.phytol.2007.10.001
Grace and Logan (2000) Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Phil Trans R Soc Lond B 355:1499–1510. doi:10.1098/rstb.2000.0710
Hakala-Yatkin M, Mäntysaari M, Mattila H, Tyystjärvi E (2010) Contributions of visible and ultraviolet parts of sunlight to photo inhibition. Plant Cell Physiol 51:1745–1753. doi:10.1093/pcp/pcq133
Hay KB, Poore AGB, Lovelock CE (2011) The effects of nutrient availability on tolerance to herbivory in a brown seaweed. J Ecol 99:1540–1550. doi:10.1111/j.1365-2745.2011.01874.x
Heller J (1996) Physic Nut. Jatropha curcas L. promoting the conservation and use of underutilized and neglected crops. Institute of Plant Genetics and Crop Plant Research and International Plant Genetic Resources Institute, Rome
Henández and Breusegem (2010) Opinion on the possible role of flavonoids as energy escape valves: novel tools for nature’s Swiss army knife? Plant Sci 179:297–301. doi:10.1016/j.plantsci.2010.06.001
Herms DA, Mattson WJ (1992) The dilemma of plant: to grow or defend. Q Rev Biol 67:283–335. doi:10.1086/417659
Hernandez I, Alegre L, Munne-Bosch S (2011) Plant aging and excess light enhance flavan-3-ol content in Cistus clusii. J Plant Physiol 168:96–102. doi:10.1016/j.jplph.2010.06.026
Huang Q, Guo Y, Fu R, Peng T, Zhang Y, Chen F (2014) Antioxidant activity of flavonoids from leaves of Jatropha curcas. Sci Asia 40:193–197. doi:10.2306/scienceasia1513-1874.2014.40.193
Jaakola L, Maatta-Riihinen K, Karenlampi S, Hohtola A (2004) Activation of flavonoid biosynthesis by solar radiation in bilberry (Vaccinium myrtillus L.) leaves. Planta 218:721–728
Kenward MG, Roger JH (1997) Small sample interference for fixed effects from restricted maximum likelihood. Biometrics 53:983–997. doi:10.2307/2533558
Khan MAM, Ulrichs C, Mewis I (2011a) Effect of water stress and aphid herbivory on flavonoids in broccoli (Brassica oleracea var. italica Plenck). J Appl Bot Food Qual 84:178–182
Khan MAM, Ulrichs C, Mewis I (2011b) Water stress alters aphid-induced glucosinolate response in Brassica oleracea var. italica differently. Chemoecology 21:235–242. doi:10.1007/s00049-011-0084.4
Khan AL, Hamayun M, Waqas M, Kang SM, Kim YH, Kim DH, Lee IJ (2012) Exophiala sp. LHL08 association gives heat stress tolerance by avoiding oxidative damage to cucumber plants. Biol Fertil Soils 48:519–529. doi:10.1007/s00374-011-0649-y
Kim YS, Hwang JW, Kim SE, Kim EH, Jeon YJ, Moon SH, Jeon BT, Park PJ (2012) Antioxidant activity and protective effects of Uncaria rhynchophylla extracts on t-BHP-induced oxidative stress in Chang cells. Biotechnol Bioprocess Eng 17:1213–1222. doi:10.1007/s12257-012-0278-9
Kumar RV, Ahlawat SP, Pandey SK, Ranjan R, Joshi DC (2012) Assessment of genetic variability in growth, reproductive phenology, seed characters and yield in Jatropha curcas (L.) genetic resources. Range Manag Agrofor 33:53–56
Lama AD, Vuorisalo T, Niemela P (2015) Global patterns of arthropod herbivory on an invasive plant, the physic nut (Jatropha curcas L.). J Appl Entomol 139:1–10. doi:10.1111/jen.12161
Lieurance D, Cipollini D (2013) Environmental influences on growth and defence responses of the invasive shrub, Lonicera maackii, to simulated and real herbivory in the juvenile stage. Ann Bot 112:741–749. doi:10.1093/aob/mct070
Loomis WE (1981) Growth and differentiation-an introduction and summary. In: Wareing PF, Phillips IDJ (eds) Growth and differentiation in plants. Pergamon Press, New York, pp 1–17
Lyytikäinen P (1994) Effects of natural and artificial defoliations on sawfly performance and foliar chemistry of Scots pine saplings. Ann Zool Fenn 31:307–318
Lyytikäinen-Saarenmaa P (1999) Growth responses of Scots pine (Pinaceae) to artificial and sawfly (Hymenoptera: Diprionidae) defoliation. Can Entomol 131:455–463
Maes WH, Trabucco A, Achten WMJ, Muys B (2009a) Climatic growing conditions of Jatropha curcas L. Biomass Bioenerg 33:1481–1485. doi:10.1016/j.biombioe.2009.06.001
Maes WH, Achten WMJ, Reubens B, Raes D, Samson R, Muys B (2009b) Plant-water relationships and growth strategies of Jatropha curcas L. seedlings under different levels of drought stress. J Arid Environ 73:877–884. doi:10.1016/j.jaridenv.2009.04.013
Marahatta S, Dangol BS, Gurung GB (2009) Temporal and spatial variability of climate change over Nepal (1976–2005). Practical Action Nepal, Kathmandu, Nepal
Massad TJ, Dyer LA, Vega GC (2012) Costs of defense and a test of the carbon–nutrient balance and growth-differentiation balance hypotheses for two co-occurring classes of plant defense. PLoS One 7:e47554–e47562. doi:10.1371/journal.pone.0047554
Miean KH, Mohamed S (2001) Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem 49:3106–3112
Moilanen J, Sinkkonen J, Salminen J-P (2013) Characterization of bioactive plant ellagitannins by chromatographic, spectroscopic and mass spectrometric methods. Chemoecology 23:165–179
Openshaw K (2000) A review of Jatropha curcas: an oil plant of unfulfilled promise. Biomass Bioenerg 19:1–15. doi:10.1016/S0961-9534(00)00019-2
Petrussa E, Braidot E, Zancani M, Peresson C, Bertolini A, Patui S, Vianello A (2013) Plant flavonoids-biosynthesis, transport and involvement in stress responses. Int J Mol Sci 14:14950–14973. doi:10.3390/ijms140714950
Prasad DMR, Izam A, Khan MdMR (2012) Jatropha curcas: plant of medicinal benefits. J Med Plants Res 6:2691–2699. doi:10.5897/JMPR10.977
Ramakrishna A, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant signal Behav 6:1720–1731. doi:10.4161/psb.6.11.17613
Rhoades DF, Cates RG (1976) Toward a general theory of plant antiherbivory chemistry. Recent Adv Phytochem 10:168–213
Samanta A, Das G, Kumar S (2011) Roles of flavonoids in plants. Int J Pharm Sci Technol 6:12–35
Scheidel U, Bruelheide H (2004) The impacts of altitude and simulated herbivory on the growth and carbohydrate storage of Petasites albus. Plant Biol 6:740–745. doi:10.1055/s-2004-830352
Selmar D (2008) Potential of salt and drought stress to increase pharmaceutical significant secondary compounds in plants. LandbauforschVoelk 58:139–144
Solovchenko AE, Merzlyak MN (2008) Screening of visible and UV radiation as a photoprotective mechanism in plants. Rus J Plant Physiol 55:719–737. doi:10.1134/S1021443708060010
Stamp N (2003) Out of the quagmire of plant defense hypotheses. Q Rev Biol 78:23–55
Wani SP, Chander G, Sahrawat KL, Rao CS, Raghvendra G, Susanna P, Pavani M (2012) Carbon sequestration and land rehabilitation through Jatropha curcas (L.) plantation in degraded lands. Agric Ecosyst Environ 161:112–120. doi:10.1016/j.agee.2012.07.028
Winkel-Shirley B (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126:485–493
Acknowledgments
Authors wish to thank the staff of the Ruissalo Botanical Garden for their cooperation and help with experiments, and Turku University Foundation for its financial support. ET was financially supported by the Academy of Finland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lama, A.D., Kim, J., Martiskainen, O. et al. Impacts of simulated drought stress and artificial damage on concentrations of flavonoids in Jatropha curcas (L.), a biofuel shrub. J Plant Res 129, 1141–1150 (2016). https://doi.org/10.1007/s10265-016-0850-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-016-0850-z