Abstract
We investigated stomatal conductance (g s) and mesophyll conductance (g m) in response to atmospheric CO2 concentration [CO2] in two primitive land plants, the fern species Pteridium aquilinum and Thelypteris dentata, using the concurrent measurement of leaf gas exchange and carbon isotope discrimination. [CO2] was initially decreased from 400 to 200 μmol mol−1, and then increased from 200 to 700 μmol mol−1, and finally decreased from 700 to 400 μmol mol−1. Analysis by tunable diode laser absorption spectroscopy (TDLAS) revealed a rapid and continuous response in g m within a few minutes. In most cases, both ferns showed rapid and significant responses of g m to changes in [CO2]. The largest changes (quote % decrease) were obtained when [CO2] was decreased from 400 to 200 μmol mol−1. This is in contrast to angiosperms where an increase in g m is commonly observed at low [CO2]. Similarly, fern species observed little or no response of g s to changes in [CO2] whereas, a concomitant decline of g m and g s with [CO2] is often reported in angiosperms. Together, these results suggest that regulation of g m to [CO2] may differ between angiosperms and ferns.
Similar content being viewed by others
Introduction
Atmospheric CO2 is a substrate for leaf photosynthesis in land plants, and thus CO2 availability at the carboxylation site is one of the most important limiting factors for leaf photosynthesis. In the process of leaf photosynthesis in C3 land plants, CO2 diffuses from the atmosphere through stomata, intercellular air spaces, and the leaf mesophyll to the site of carboxylation in the chloroplasts. CO2 concentration in the chloroplast is lower than that in the atmosphere because of significant resistance to CO2 diffusion through this diffusional pathway, i.e., limitations in CO2 diffusion strongly reduce leaf photosynthesis. There are two major CO2 diffusional limitations; CO2 conductance though stomata, g s, and that from substomatal cavities to the chloroplast, termed g m.
Atmospheric CO2 levels have changed substantially over the evolutionary history of land plants. It is estimated that atmospheric CO2 levels were approximately 10 times higher than the present when land plants started to evolve 360–480 million years ago (Royer et al. 2004). Ferns are a major component of the fossil flora, and although they are primitive, non-seed plants, they are closely related to seed plants (Pryer et al. 2001). Atmospheric CO2 levels fell abruptly during the Cretaceous period (Kuypers et al. 1999), which coincides with a major diversification in the fern group (Pryer et al. 2004). On the other hand, angiosperms, which are currently the dominant group of seed plants, emerged during a period when atmospheric CO2 level was only two- to three-fold higher than the present (Haworth et al. 2011). This implies that ferns and angiosperms evolved under different selection pressures, which may have resulted in different mechanisms of CO2 diffusion between these two plant groups (Carriquí et al. 2015). Changes in the mechanisms of CO2 diffusion in plant evolutionary history have been suggested because stomatal frequency in fossil plants, which strongly affects g s, has been shown to track changes in atmospheric CO2 level (Woodward 1998). As such, it’s suggested that stomatal function developed to enhance CO2 diffusion to cope with decreases in CO2 level. However, changes in g m in land plant history cannot be determined through similar anatomical imprints in the fossil record. Although the difference in g m is possibly still partially reflected in extant plants of angiosperms and ferns. Leaf mesophyll anatomy affecting g m, including chloroplast surface area facing the intercellular airspaces and cell wall thickness, could have changed from ferns to angiosperms (Carriquí et al. 2015), which may be affected by the decrease in atmospheric CO2 level. A comparison of CO2 diffusional limitations in extant ferns with extant angiosperms could provide crucial information to estimate how photosynthesis traits have evolved in land plants. If atmospheric CO2 levels can influence selection pressure, phylogenetically distant fern groups may also vary in internal morphology and g m.
In the present atmospheric CO2 conditions, fern species have much lower photosynthetic capacity than angiosperms (Wright et al. 2005). In ferns, both g s and g m are lower than in angiosperms. A lower g m is suggested to be the major mechanism underlying the lower photosynthetic capacity of fern species (Carriquí et al. 2015). However, there are only three published determinations of g m of fern species to the best of our knowledge (Carriquí et al. 2015; Gago et al. 2013; Volkova et al. 2009). Anatomical and physiological mechanisms underlying the low g m of fern species still remain to be confirmed.
The response of g s to atmospheric CO2 concentration [CO2] is different between angiosperms and ferns. Extensive studies on angiosperms have shown that g s typically increases with a decrease in [CO2] (e.g., Brodribb et al. 2009; Messinger et al. 2006). However, three ferns, Osmunda regalis, Blechnum gibbum and Nephrolepis exaltata, showed small responses to changes in [CO2] (Gago et al. 2013). The averaged g s for six ferns and lycophytes showed no response to an increase in [CO2] above ambient, while they showed a slight increase with a decrease in [CO2] (Brodribb et al. 2009). Studies for the response of g m to [CO2] are limited compared with g s in angiosperms, and to the best of our knowledge, there is only one published study on the response of g m to [CO2] in fern species (Gago et al. 2013). For angiosperms, there is conflicting evidence as to how g m responds to [CO2]. Some studies reported insignificant effects of [CO2] on g m (Harley et al. 1992; Tazoe et al. 2009), whereas other studies reported a decline in g m at high [CO2] (Bunce 2010; Douthe et al. 2011; Hassiotou et al. 2009; Loreto et al. 1992, Tazoe et al. 2011), or showed curved responses to changes in [CO2] (Flexas et al. 2007; Vrábl et al. 2009). Gago et al. (2013) obtained a curved response in g m with changes in [CO2] for three fern species. However, the observed decline in g m at low [CO2] in angiosperms and ferns (sub-stomatal CO2 concentration, C i < 50 µmol mol−1) may be an artifact related to partially photorespired CO2 (Tholen et al. 2012). Furthermore, the chlorophyll fluorescence technique used can lead to errors in the estimation of g m in conditions of changing [CO2] (Gilbert et al. 2011). Because of these potential artifacts and errors, it is necessary to confirm previous studies on the response of g m to [CO2] in angiosperms and ferns through the use of complimentary methods. We chose specifically to look at ferns because of the limited information published on CO2 responses and to determine if like stomatal responses to CO2, ferns also differed in g m responses compared with angiosperms.
The purposes of this study were to determine: (1) the photosynthetic traits of ferns, including g m and g s at the present [CO2] (400 µmol mol−1) for comparison against published values for ferns and angiosperms, and (2) the rapid and continuous response of g m, g s and photosynthetic rate of ferns to changing [CO2]. For these purposes, we developed a custom-designed gas exchange system using a concurrent measurement of gas exchange and carbon isotope ratio using tunable diode laser absorption spectroscopy (TDLAS), to quantify the rapid, continuous responses in g m in fern species in response to changes in [CO2] with a time resolution of a few minutes (Tazoe et al. 2009, 2011). O2 gas was used at a level of 2 % for gas exchange measurements in order to minimize the effect of photorespiration on carbon isotope measurements. To the best of our knowledge, this is the first study to examine continuous responses in g m in fern species in response to changes in [CO2]. We also determined leaf anatomical traits using light micrographs and calculated photosynthetic parameters using the light–response curve and A/C i curve, to compare the photosynthetic traits of ferns with those of angiosperms reported previously.
We selected two fern species from order Polypodiales, Pteridium aquilinum and Thelypteris dentata. From recent phylogenetic studies, Polypodiales is the most modern order among the seven fern orders in Polypodiopsida (Smith et al. 2006). The estimated divergence time of Pteridium (Dennstaedtiaceae family) and Thelypteris (Thelypteridiaceae family, Eupolipods II) is ~90 and ~65 million years, respectively (Pryer et al. 2004), when atmospheric CO2 levels decreased with time from ~2,000 to ~500 ppm (Bice and Norris 2002). P. aquilinum was possibly distributed worldwide in the Oligocene (Der et al. 2009) when the atmospheric CO2 levels had decreased (~400 ppm; Zhang et al. 2013). P. aquilinum and T. dentata grow in open sites and show higher photosynthetic rates than those of other ferns that grow in shady sites. High photosynthetic rate assures high accuracy in the estimation of g m using the carbon isotope method.
Materials and methods
Plants materials
Pteridium aquilinum (L.) Kuhn (Fig. 1a) and T. dentata (Forssk.) E. P. St. John (Fig. 1b) were used. P. aquilinum is a deciduous fern that grows in open habitats and is distributed widely in temperate zones in the Northern hemisphere. T. dentata is an evergreen fern that grows in open habitats in tropical or subtropical zones, and which has recently expanded into southern coastal areas in Japan (Murakami et al. 2007). Rhizomes of P. aquilinum and T. dentata were purchased commercially (Takayama Engei, Kyoto, Japan) and collected around the greenhouse at Kyoto Institute of Technology (Ukyo-ku, Kyoto, Japan), respectively. Five rhizomes of each species were planted in 3-liter pots filled with mixed soil (peat moss:humus:sand = 3:3:1 volume ratio) in a 50 % shaded glasshouse. Five plants of both species were used for light–response curve, A/C i curve, and anatomical analysis. Three or four of the five plants were used for CO2 response measurements. Average daytime photosynthetic photon flux density (PPFD) in the glasshouse was 221 ± 7 µmol m−2 s−1. Plants were watered every 2 days, fertilized with a 1/2,000 solution of Hyponex 6-10-5 (Hyponex Japan, Osaka, Japan) once a month. The gas exchange experiment was carried out in October 2013. Average temperature and relative humidity in the glasshouse from frond emergence to the experiments were 20.7 ± 0.1 °C and 74.5 ± 0.3 %, respectively.
Estimation of g m
Gas exchange and carbon isotope discrimination were measured concurrently using a custom-designed system constructed at the National Institute for Agro-Environmental Sciences (Fig. 2), which was based on previous studies (Nelson et al. 2008; Tuzson et al. 2008; Wada et al. 2011). A custom-made gas exchange system was connected to a CO2 isotope analyzer, a tunable diode laser absorption spectroscope (QC-TILDAS-ISO, Aerodyne Research Inc., Billerica, MA, USA) for the sequential measurement of CO2 isotopologues. A custom leaf chamber with a diameter of 12 cm with temperature and humidity controlled by a thermo chiller (SMC, HRS018-AF-10) and a bubbler, respectively, was connected to the CO2/H2O analyzer (Li-7000, LI-COR) for the gas exchange measurements. Leaf area was measured using scanned images of the cut leaves immediately after the measurement, using ImageJ software (http://imagej.nih.gov/ij/). We measured the leaf boundary layer conductance in accordance with the leaf area by obtaining a calibration curve using saturated filter papers with different areas. The fan placed inside the chamber mixed the air in the chamber completely. A thermocouple placed inside the chamber was connected to the gas exchange analyzer in order to record leaf temperature. A red and blue light emitting diode (LED) light source (red: blue 8:1, LEDRB-630DL, Opto Code Corp., Tokyo, Japan) was set onto the chamber, with PPFD of 500 µmol m−2 s−1 at the leaf surface. The flow rate was set at 500 ml min−1, and leaf temperature at 25–28 °C. Vapor pressure deficit (VPD) was set at <1.5 kPa. N2 and O2 gas were mixed using mass controllers (SEC-E40, HORIBA Ltd., Kyoto, Japan) to generate 2 % O2. To determine photosynthetic responses to changes in atmospheric CO2 concentration [CO2], a fully expanded mature leaf was clamped into the chamber, and the ambient CO2 concentration (C a) was kept at 400 µmol mol−1 for 40–60 min. After that, C a was first reduced to 200 µmol mol−1 for 80 min, and then increased to 700 μmol mol−1 for 80 min. Finally, C a was decreased to 400 µmol mol−1. We varied C a from 200 to 700 μmol mol−1 because leaf photosynthesis of ferns was CO2-limited in this range of C a in A/C i curve analysis. Measurements were performed at 30 s intervals, calibrated every 30 min using two standard gas cylinders, 200 and 700 μmol mol−1, during the measurement (Fig. 3). Stability of TDLAS was tested using standard CO2 gas at a CO2 concentration of 400 μmol mol−1 for 2 h before and after the measurements. Analysis with Allan variance showed that deviation of δ13C for 30 min was <0.03 %. The δ13C of the gas was stabilized completely within 10 min. Observed carbon isotope discrimination during photosynthesis (\(\delta_{o}\)) was calculated using the following equation (Evans et al. 1986),
where δ 13 C a and δ 13 C ref are the carbon isotope composition in the leaf chamber and in reference air. ξ = C ref/(C ref – C a), where C a and C ref are the CO2 concentration in the leaf chamber and in reference air. ξ was kept at <9 during measurements in order to ensure high precision and accuracy for g m estimation (Pons et al. 2009). Mesophyll conductance was calculated using the equations reported by Evans and von Caemmerer (2013) assuming no photorespiration:
\(t =\left( { 1 { + }a'} \right)E/2g_{\text{ac}}^{t}\), where a′ is a combined fractionation factor through the boundary layer and stomata,
where a b (2.9 ‰) and a (4.4 ‰) are the fractionation through CO2 diffusion in the boundary layer and air, respectively (Evans et al. 1986). C s and C i are the CO2 concentration at the leaf surface and in the leaf intercellular air space, respectively. E is the transpiration rate, and g tac is total conductance to CO2 diffusion. b (30 ‰) is the fractionation associated with Rubisco carboxylation (Roeske and O’Leary 1984), a i (1.8 ‰) is the fractionation factor for dissolution and diffusion through water (O’Leary 1981), and R d is day respiration. The parameter e, which is associated with day respiration, was calculated as e = δ 13 C tank − δ 13 C atmosphere, assuming no fractionation by day respiration (Evans and von Caemmerer 2013; Tazoe et al. 2009). δ 13 C tank was from −34 to −36 ‰, and δ 13 C atmosphere was assumed to be −8 ‰. \(\delta_{i}\) is fractionation when C i = C c without respiratory fractionation:
\(\delta_{e}\) is fractionation with respiration, which was calculated as:
Γ * is the CO2 compensation point in the absence of R d, which was estimated following the procedure reported by Laisk et al. (1984). We used C*, the apparent CO2 compensation point provided by Laisk et al. (1984), as a proxy of Γ * (Douthe et al. 2011). C * was 65.1 ± 3.9 and 65.1 ± 4.4 μmol mol−1 in P. aquilinum and T. dentata, respectively, and R d was 3.8 ± 0.2 and 3.4 ± 0.2 μmol m−2 s−1, respectively.
An example of the 80 min cycle of the measurement of carbon isotope ratio using tunable diode laser absorption spectroscopy, where atmospheric CO2 concentration [CO2] was altered from 200 to 700 μmol mol−1. After calibration gas 1 (std 1) was measured, sample and reference gas from the Li-7000 was measured at a reference CO2 of 200 μmol mol−1, followed by the measurement of calibration gas 2 (std 2). Thereafter, the sample gas was measured at a reference CO2 of 200 μmol mol−1 again, and then the reference CO2 was changed to 700 μmol mol−1 and then sample gas was measured. A similar measurement cycle was repeated for the changes in [CO2] from 700 to 400 μmol mol−1 and from 400 to 200 μmol mol−1. 118 × 113 mm (300 × 300 DPI)
Estimation of photosynthetic parameters
Leaf photosynthetic parameters were estimated from the light–response curve model (Ogren and Evans 1993) and the A/C i curve fitting method (A/C i Curve Fitting 10.0.xls, http://landflux.org/Tools.php, Ethier and Livingston 2004; Ethier et al. 2006) using a photosynthesis system (Li-6400, LI-COR). Leaf temperature and vapor pressure deficit (VPD) were set at 25 °C and 1.5 kPa, respectively. C a was 400 μmol mol−1 for the light–response curve analysis. PPFD was decreased stepwise from 500 to 0 μmol m−2 s−1. Thereafter, PPFD was returned to 400 μmol m−2 s−1 and then increased stepwise to 1,500 μmol m−2 s−1. Light-saturated photosynthesis rate (A sat), curvature factor, quantum use efficiency, dark respiration rate, light compensation point, and g s were obtained from light–response curves. For the A/C i curve analysis, PPFD was set at 1,000 and 500 μmol m−2 s−1 for P. aquilinum and T. dentata, respectively, because our previous experiment obtained saturated PPFD of 1,000 and 500 μmol m−2 s−1 for P. aquilinum and T. dentata, respectively. C a was decreased stepwise from 400 to 50 μmol mol−1, then returned to 400 μmol mol−1, and increased stepwise to 2,000 μmol mol−1. Maximum carboxylation rate (V cmax) and electron transport rate (J) were calculated from the A/C i curve, assuming constant g m during the changes in C i.
Analysis of leaf morphological traits
Leaf mass per area was calculated as leaf dry weight divided by leaf area. Leaf mesophyll anatomy was determined using light and transmission electron micrographs. Leaf sections of 2 × 3 mm were fixed in 5 % glutaraldehyde and 1 % osmium tetroxide, and were embedded in Spurr’s resin (Low Viscosity Resin kit, TAAB, Aldermaston, UK). Transverse Sects. (800 nm thick) were stained with 1 % toluidine blue solution. Anatomical characteristics were determined from digitized images of micrographs taken at ×400 magnification (BX51-33, OLYMPUS, Tokyo, Japan). The surface area of mesophyll cells and chloroplasts exposed to intercellular air spaces per unit leaf area (S mes and S c) were estimated for transverse sections as described by Hanba et al. (2002). Transverse Sects. (70 nm thick) were stained with 2 % uranyl acetate and Reynold’s lead citrate. Thickness of cell walls covered with chloroplasts (cell wall thickness), and chloroplast thickness and width were measured from ×6,000 and ×2,500 magnification images, respectively, from micrographs taken by a transmission electron microscope (JEM-1220, JOEL, Tokyo, Japan), analyzed using ImageJ software (http://imagej.nih.gov/ij/).
Statistical analysis
Differences in mean values between species were tested using an unpaired t test to analyze photosynthetic parameters and leaf morphological traits. The effect of [CO2] on leaf gas exchange was analyzed using an unpaired t test. These statistical analyses were conducted using EZR version 1.24 (Kanda 2013; http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html).
Results
Photosynthesis rate (A) was light saturated at a PPFD of 500 μmol m−2 s−1 for both P. aquilinum and T. dentata (Fig. 4a). For T. dentata, A tended to decrease when PPFD exceeded 1,000 μmol m−2 s−1. There were no significant differences in A sat, curvature factor, quantum use efficiency, respiration rate, or light compensation point between the two species (Table 1). When PPFD decreased from 500 to 0 μmol m−2 s−1, g s in P. aquilinum tended to decrease but that of T. dentata was almost constant (Fig. 4b), with a significant increase in intercellular CO2 concentration (C i) in both species (P < 0.05; Fig. 4c). The g s of P. aquilinum was compared with that of T. dentata and showed no significant difference at a PPFD of 2,000 μmol m−2 s−1. V max and J calculated using A/C i curves were also not significantly different between the two species (Fig. 5a; Table 1). When C i was decreased from 300 to 50 μmol mol−1, the g s of both species significantly increased (P < 0.05; Fig. 5b). With C i of 400 μmol mol−1 to 1,800 μmol mol−1, g s values remained almost constant in both species.
Changes in a leaf photosynthesis rate (A), b stomatal conductance (g s), and intercellular CO2 concentration (C i), c against photosynthetic photon flux density (PPFD) in Pteridium aquilinum (filled circles) and Thelypteris dentata (open circles) at 400 μmol mol−1 of ambient atmospheric CO2. Data points are means with bars for standard errors (n = 5). 81 × 156 mm (300 × 300 DPI)
Response of a photosynthesis rate (A) and b stomatal conductance (g s) to intercellular CO2 concentration (C i) in Pteridium aquilinum (filled circles) and Thelypteris dentata (open circles). Ambient CO2 concentration was first decreased from 400 to 50 μmol mol−1, then returned to 400 μmol mol−1 and increased to 2000 μmol mol−1. Data points are means with bars for standard errors (n = 5)
Transverse sections of the fronds of P. aquilinum (Fig. 6a, c) and T. dentata (Fig. 6b, d) showed that both ferns had loosely packed mesophyll cells and lacked distinct palisade tissue. Both ferns also had chloroplasts in the upper and lower epidermal cells. The width and thickness of chloroplasts in T. dentata were significantly larger than those of P. aquilinum (Table 2). The chloroplasts had large spaces between them and, as a result, 59 and 44 % of S mes were not covered with chloroplasts for P. aquilinum and T. dentata, respectively. The S mes and internal air spaces of P. aquilinum were significantly larger than those of T. dentata (Table 2). Other traits including S c, LMA, leaf thickness, cell wall thickness, and chloroplast width/thickness were not significantly different between the two species.
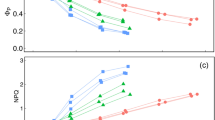
When atmospheric CO2 concentration [CO2] was decreased from 400 to 200 μmol mol−1, g s of both ferns increased slightly with time (Fig. 7a), with a 3.7 mmol m−2 s−1 increase in P. aquilinum and a 5.4 mmol m−2 s−1 increase in T. dentata on average (Table 3). In contrast, g m of both ferns decreased rapidly (Fig. 7d), with a 8.6 mmol m−2 s−1 decrease in P. aquilinum and a 2.1 mmol m−2 s−1 decrease in T. dentata (Table 3). A and C i of P. aquilinum and T. dentata also showed rapid decreases of 5.1 and 3.2, 134.2, and 150.8 μmol m−2 s−1, respectively (Fig. 7 g, j). The decrease in C c was 47 and 65.1 μmol mol−1 in P. aquilinum and T. dentata, respectively (Fig. 7 m).
Changes in stomatal conductance (g s), mesophyll conductance (g m), photosynthesis rate (A), intercellular CO2 concentration (C i) and chloroplast CO2 concentration (C c) in response to atmospheric CO2 concentration [CO2] for Pteridium aquilinum (filled circles) and Thelypteris dentata (open circles). [CO2] was first decreased from 400 to 200 μmol mol−1 (left panels), then increased from 200 to 700 μmol mol−1 (middle panels), and finally decreased from 700 to 400 μmol mol−1 (right panels). Data points were averaged for two data (1 min) from 3 or 4 different plants with bars for standard errors (n = 3 or 4). 167 × 196 mm (300 × 300 DPI)
When [CO2] was increased from 200 to 700 μmol mol−1, g s did not change in either species (Fig. 7b; Table 3). g m of P. aquilinum decreased by 1.1 mmol m−2 s−1, whereas it increased by 4.9 mmol m−2 s−1 in T. dentata (Table 3; Fig. 7e). A and C i increased rapidly, by 8 and 6.8 μmol m−2 s−1 and 405.5 and 414.9 μmol mol−1 for P. aquilinum and T. dentata, respectively (Fig. 7 h, k), and the respective increases in C c were 175 and 254.5 μmol mol−1 (Fig. 7n).
When [CO2] was decreased from 700 to 400 μmol mol−1, g s of both species increased slightly with time (Fig. 7c), with a 8.3 mmol m−2 s−1 increase in P. aquilinum and a 0.9 mmol m−2 s−1 increase in T. dentata on average (Table 3). The g m of P. aquilinum increased by 6.5 mmol m−2 s−1, whereas that of T. dentata decreased by 6.6 mmol m−2 s−1 on average (Table 3; Fig. 7f). A and C i decreased rapidly, by 4.0 and 3.2 and 255.4 and 266.6 μmol mol−1 for P. aquilinum and T. dentata, respectively (Fig. 7i, l), and the respective decreases in C c were 115.1 and 213.2 μmol mol−1 (Fig. 7o).
Discussion
Steady-state leaf photosynthetic traits and morphology in ferns
There was no significant difference in photosynthesis traits between P. aquilinum and T. dentata obtained from the analysis of light–response curves and A/C i curves (Table 1). P. aquilinum and T. dentata are Polypodiales, which is the most modern among the fern orders (Smith et al. 2006). Photosynthesis traits were compared with those reported in previous studies of fern species in Polypodiales. A sat, dark respiration rate, light compensation point, V max, and J were within the range of those reported in previous studies, although the growth conditions differed (Carriquí et al. 2015; Gago et al. 2013; Sessa and Givnish 2014). At present, there is little evidence that photosynthetic traits are different between phylogenetically distant fern groups; two basal ferns Equisetum telmateia (Equisetales) and O. regalis (Osmundales) had photosynthetic traits within the range of those in Polypodiales (Carriquí et al. 2015; Gago et al. 2013). However, photosynthesis data for basal ferns are so scarce that further systematic studies are needed for phylogenetic consideration. The present result confirmed that the photosynthetic capacity of ferns is generally lower than angiosperms; ferns had lower A sat, dark respiration rate, g s, V max, and J than angiosperms (Carriquí et al. 2015). The values reported in this study are in the range of those reported in Carriquí et al. (2015) for fern species.
Although photosynthesis traits are similar between P. aquilinum and T. dentata, internal air spaces and S mes were larger in P. aquilinum than in T. dentata, because of its loosely packed mesophyll cells. The smaller size of chloroplasts in P. aquilinum (Table 2) offset the effect of higher S mes, which involves similar S c between P. aquilinum and T. dentata. This similar S c may relate to the similar photosynthetic traits between species in the present study. Compared with angiosperms, where the lowest S c so far reported was 5.0 m2 m−2 in a tree species Acer rufinerve grown in shade (Hanba et al. 2001), the S c measurements of fern species here were among the lowest values, with an S c of 3.9 ± 0.6 m2 m−2 in P. aquilinum and 5.0 ± 0.4 m2 m−2 in T. dentata. Terashima et al. (2006) reported that in seed plants, the average S c was 15.01 m2 m−2 in annuals, 12.05 m2 m−2 in deciduous trees, and 14.45 m2 m−2 in evergreen trees. Carriquí et al. (2015) reported that the average S c for seven angiosperms and ferns was 10.3 and 7.6 m2 m−2, respectively. These previous studies, together with our results, indicate that the S c of ferns is lower than the S c of most angiosperms. Small S c measurements in fern species are related to small mesophyll thickness with large intercellular airspaces, and may also be partly affected by the size of chloroplasts (Table 2). S c is one of the most significant factors affecting g m, where high S c allows plants to increase diffusion of CO2 into chloroplasts. Angiosperms had much lower atmospheric CO2 levels than ferns at their emergence period, and this may have been crucial for angiosperms to increase diffusional surface for CO2 to achieve high photosynthetic rates.
Cell wall thickness was 0.23 ± 0.02 μm in P. aquilinum (Table 2), which is similar to the cell wall thickness of 0.194 μm in P. aquilinum reported by Carriquí et al. (2015). The cell wall thickness of P. aquilinum and T. dentata obtained here (0.23 ± 0.02 μm and 0.30 ± 0.03 μm) are in the range of typical values for deciduous trees (0.2–0.3 μm; Terashima et al. 2006). Carriquí et al. (2015) reported higher averaged values of cell wall thickness in ferns (0.359 μm) than those in angiosperms (0.251 μm) and suggested an evolutionary trend towards reduced thickness from ferns to angiosperms. More evidence is needed to substantiate this evolutionary trend, considering the uncertainties in estimation of cell wall thickness using electron micrographs and large variability among the same phylogenic group (from 0.194 to 0.687 μm in ferns, Carriquí et al. 2015). LMAs of P. aquilinum and T. dentata were much smaller than those in tree species (Hanba et al. 1999; Tomás et al. 2013), which may also be partly affected by the low mesophyll thickness in ferns.
Steady-state g m of fern species compared with angiosperms at the present [CO2]
One of the goals of our study was to determine steady-state g m values of ferns and compare them with the published values for angiosperms at the present [CO2] (400 μmol mol−1). For the estimation of g m, carbon isotope, chlorophyll fluorescence, and A/C i curve-fitting methods have all been used previously (Pons et al. 2009). There are only three studies that reported the g m of ferns. Gago et al. (2013) showed that the g m of O. regalis, B. gibbum and N. exaltata were between 30 and 73 mmol m−2 s−1 using a chlorophyll fluorescence method, and between 30 and 112 mmol m−2 s−1 using an A/C i curve-fitting method. Volkova et al. (2009) showed that g m of Dicksonia antarctica grown in the shade and at high irradiance was 115 ± 35 and 155 ± 64 mmol m−2 s−1, respectively, estimated from A/C i curve fitting. Carriquí et al. (2015) reported that g m varies from 26 to 253 mmol m−2 s−1 for seven fern species using a chlorophyll fluorescence method. We used a different method from previous studies reporting the g m of ferns (Carriquí et al. 2015; Gago et al. 2013; Volkova et al. 2009). The g m values in the present study (35.6 to 52.5 mmol m−2 s−1) were among the lowest values reported from these three previous studies. Furthermore, the g m values of P. aquilinum and T. dentata in the present study were lower than those of typical seed plants, including angiosperms and gymnosperms (Flexas et al. 2008, 2012).
The cause of the low g m in fern species remains to be clarified, but some anatomical traits have been suggested to play a role (Gago et al. 2013; Carriquí et al. 2015). As previously described, S c values in P. aquilinum and T. dentata (3.9 ± 0.6 and 5.0 ± 0.4 m2 m−2) were lower than those of most seed plants including annuals (15.0 m2 m−2), deciduous broadleaved trees (12.1 m2 m−2), and evergreen trees (14.5 m2 m−2) (Terashima et al. 2006). When anatomical traits in the present study were compared with those of an angiosperm, Lysimachia minoricensis that had similar LMA (31.4 g m−2) to our study (31.7 and 39.7 g m-2), the cell wall thickness was similar (0.213 μm and 0.21–0.28 μm), but S c was much smaller in the present study (3.9 and 5.0 m2 m−2) than L. minoricensis (8.9 m2 m−2; Carriquí et al. 2015). Therefore, the low g m of fern species may be at least partly affected by low S c; a significant positive correlation was obtained between g m and S c, for angiosperms (Terashima et al. 2006, 2011). The concentration and activities of membrane proteins that transport CO2 into mesophyll cells, such as aquaporins (Hanba et al. 2004; Kawase et al. 2013; Terashima and Ono 2002), could also account for the low g m of ferns.
Dynamic response of g s and g m in response to changes in [CO2]
The slight increase in g s (<10.4 %) followed a decrease in C a from 400 to 200 μmol mol−1 (Table 3; Fig. 7). This was consistent with Brodribb et al. (2009), who reported that six ferns/lycopods showed small increases in g s when C a decreased from 380 to 100 μmol mol−1. In a study on B. gibbum and O. regalis, Gago et al. (2013) reported a slight decrease in g s when C a decreased from 400 to 50 μmol.mol−1, and a similar result was obtained in our study (Fig. 5b). No change was observed in g s after an increase in C a (from 200 to 700 μmol mol−1). This result supports Brodribb et al. (2009), who reported no significant changes in g s when C a was increased from 380 to 600 μmol mol−1 for three Polypodiales ferns. The insensitivity of stomata to the increase in C a contrasts with the response seen in angiosperms, which showed a significant decrease (Brodribb et al. 2009). Although the physiological control of stomatal response to [CO2] in angiosperms remains poorly understood, Brodribb et al. (2009) hypothesized that a signaling pathway between mesophyll and guard cells in response to [CO2] may be present in angiosperms but not in ferns. However, the slight increase in g s in ferns following decreased C a, is similar (but less distinct) to the response in angiosperms, suggesting that the mechanisms involved in CO2 sensing in angiosperms may be partly functional in ferns.
In contrast to the increase in g s, the g m of the two fern species in the present study decreased quickly when C a was decreased from 400 to 200 μmol mol−1 (Fig. 7d). Decreased g m at low C i (<200 μmol mol−1) has been reported for some angiosperms (Flexas et al. 2007; Vrábl et al. 2009) and for three ferns (Gago et al. 2013) using chlorophyll fluorescence or isotope methods. However, previous studies pointed out that the decrease in g m at low C i might be because of artifacts caused by respiration and photorespiration, when measurements were performed at atmospheric O2 level (20 %, Gago et al. 2013; Tholen et al. 2012). As far as we know, three previous studies have been conducted to estimate the g m response to [CO2] using 2 % or 1 % O2 to minimize the effect of photorespiration (Mizokami et al. 2015; Tazoe et al. 2009, 2011). Tazoe et al. (2009, 2011) reported that in some angiosperms, g m did not decrease significantly in response to an instantaneous reduction of C i. Mizokami et al. (2015) reported that in Nicotiana plumbaginifolia, both g m and g s decreased with C i on the application of abscisic acid (ABA), but they increased in the absence of ABA, which suggested that g s has an effect on g m and its response to [CO2] (Tazoe and Santrucek 2015). In our study, however, the rapid decrease in g m after decreasing C a is in contrast to the gradual and slight increase in g s for two fern species (Fig. 7a, d). The effect of photorespiration was minimized, because we measured gas exchange at 2 % O2. The present result suggested that the decrease in g m at 200 μmol mol−1 of C a in the two ferns may be independent of g s.
The g m did not decrease from 200 to 700 μmol mol−1 with [CO2] for T. dentata and P. aquilinum (Fig. 7e; Table 3), which is in contrast to the results that reported significant decreases in g m from 200 to 1,000 μmol mol−1 of [CO2] for three angiosperms (Tazoe et al. 2011). Although a trend where g m declines at high [CO2] is frequently reported in angiosperms (Bunce 2010; Douthe et al. 2011; Flexas et al. 2007; Mizokami et al. 2015; Vrábl et al. 2009) and also in ferns (Gago et al. 2013), the dependence of g m on CO2 varied between studies and species. g m was highest at <200 μmol mol−1 of C i in O. regalis, whereas N. exaltata showed highest g m at C i of 400 μmol mol−1 (Gago et al. 2013). These results suggest that plant species have “optimum” g m at different CO2 levels, which might reflect atmospheric CO2 levels during their evolution. P. aquilinum showed its highest g m at [CO2] of 400 μmol mol−1 (Table 3), with this species distributed worldwide in the Oligocene (Der et al. 2009) when the CO2 level had decreased near to the present level (~400 ppm; Zhang et al. 2013). T. dentata showed its highest g m at [CO2] of 700 μmol mol−1 (Table 3), and the estimated divergence time of T. dentata is ~65 million years (Pryer et al. 2004) when CO2 levels were high (~1,000 ppm; Bice and Norris 2002).
The physiological mechanisms for g m responses to changes in [CO2] have not been identified for angiosperms or ferns. Mizokami et al. (2015) suggested that the decrease in g m in response to the increase in [CO2] may be mediated by inactivation of the plasma membrane intrinsic proteins via carbonic anhydrase activity. However, an increased g m in response to increased [CO2] in the present study (Fig. 7e) suggested that, in T. dentata, other mechanisms may be involved. Terashima et al. (2011) suggested that porosity and tortuosity of the cell wall, or diffusion of HCO3 − could be affected by pH via changes in [CO2], and thus affect CO2 diffusion through cell walls. Irrespective of the mechanisms for g m, g m imposes major photosynthetic limitations in ferns (Carriquí et al. 2015). This is a major reason why the g m response of ferns to [CO2] has a large effect on the response of photosynthesis (A) to [CO2] (Fig. 7 g–i). A decrease in g m in response to low [CO2] is clearly not advantageous for photosynthesis in the present low atmospheric CO2 environment because low g m (Fig. 7d) causes low C c (Fig. 7 m) and thus a diminished rate of photosynthesis (Fig. 7). The regulation mechanism of g m with changes in [CO2] may have developed with plant evolution in response to historical changes in atmospheric CO2 levels and g s.
Conclusion
In a steady-state with the present level of CO2 in the environment (400 μmol mol−1), two ferns P. aquilinum and T. dentata had lower mesophyll conductance (g m) than the angiosperms measured so far, which may be partly imposed by the small S c observed. The dynamic response of g s to changes in [CO2] confirmed previous studies that reported slight increases in g s after changes in [CO2] from the present level to below the present level (e.g., 200 μmol mol−1) for fern species, in which the sensitivity of g s to the decrease in [CO2] was much lower than in angiosperms. Although a causal mechanism for the CO2 response of g m still remains to be clarified, the dynamic response in g m showed a rapid and significant decrease to decreased [CO2] (from 400 to 200 μmol mol−1), which was in contrast to the response of angiosperms. These dynamic CO2 responses in g s and g m in P. aquilinum and T. dentata suggest that fern species have not evolved efficient regulatory mechanisms to cope with the low CO2 levels. Future studies of the CO2 response of g m and g s in ferns, and studies of g m in other primitive plants, such as mosses, will be helpful to further elucidate how photosynthetic traits have evolved in the history of land plants.
References
Bice KL, Norris RD (2002) Possible atmospheric CO2 extremes of the Middle Cretaceous (late Albian–Turonian). Paleoceanography 17:1070
Brodribb TJ, McAdam SAM, Jordan GJ, Feild TS (2009) Evolution of stomatal responsiveness to CO2 and optimization of water—use efficiency among land plants. New Phytol 183:839–847
Bunce JA (2010) Variable responses of mesophyll conductance to substomatal carbon dioxide concentration in common bean and soy bean. Photosynthetica 48:507–512
Carriquí M, Cabrera HM, Conesa MÀ, Coopman RE, Douthe C, Gago J, Gallé A, Galmés J, Ribas-Carbo M, Tomás M, Flexas J (2015) Diffusional limitations explain the lower photosynthetic capacity of ferns as compared with angiosperms in a common garden study. Plant Cell Environ 38:448–460
Der JP, Thomson JA, Stratford JK, Wolf PG (2009) Global chloroplast phylogeny and biogeography of bracken (Pteridium; Dennstaedtiaceae). Am J Bot 96:1041–1049
Doi M, Shimazaki K (2008) The stomata of the fern Adiantum capillus-veneris do not respond to CO2 in the dark and open by photosynthesis in guard cells. Plant Physiol 147:922–930
Douthe C, Dreyer E, Epron E, Warren CR (2011) Mesophyll conductance to CO2, assessed from online TDL–AS records of 13CO2 discrimination, displays small but significant short–term responses to CO2 and irradiance in Eucalyptus seedlings. J Exp Bot 62:5335–5346
Ethier GJ, Livingston NJ (2004) On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar–von Caemmerer-Berry leaf photosynthesis model. Plant Cell Environ 27:137–153
Ethier GJ, Livingston NJ, Harrison DL, Black TA, Moran JA (2006) Low stomatal and internal conductance to CO2 versus Rubisco deactivation as determinants of the photosynthetic decline of ageing evergreen leaves. Plant Cell Environ 29:2168–2184
Evans JR, von Caemmerer S (2013) Temperature response of carbon isotope discrimination and mesophyll conductance in tobacco. Plant Cell Environ 36:745–756
Evans JR, Sharkey TD, Berry JA, Farquhar GD (1986) Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Aust J Plant Physiol 13:281–292
Flexas J, Diaz-espejo A, Galmés J, Kaldenhoff R, Medrano H, Ribas-carbó M (2007) Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant Cell Environ 30:1284–1298
Flexas J, Ribas-Carbó M, Diaz-Espejo A, Galmés J, Medrano H (2008) Mesophyll conductance to CO2: current knowledge and future prospects. Plant Cell Environ 31:602–621
Flexas J, Barbour MM, Brendel O, Cabrera HM, Carriquí M, Díaz-Espejo A, Douthe C, Dreyerc E, Ferrio JP, Gago J, Gallé A, Galmés J, Kodama N, Medrano H, Niinemets Ü, Peguero-Pina JJ, Pou A, Ribas-Carbó M, Tomás M, Tosens T, Warren CR (2012) Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Sci 193–194:70–84
Gago J, Coopman RE, Cabrera HM, Hermida C, Molins A, Conesa MÀ, Galmés J, Ribas-Carbó M, Flexas J (2013) Photosynthesis limitations in three fern species Physiol Plantarum 149:599–611
Gilbert ME, Pou A, Zwieniecki MA, Holbrook NM (2011) On measuring the response of mesophyll conductance to carbon dioxide with the variable J method. J Exp Bot 63:413–425
Hanba YT, Miyazawa SI, Terashima I (1999) The influence of leaf thickness on the CO2 transfer conductance and leaf stable carbon isotope ratio for some evergreen tree species in Japanese warm–temperate forests. Funct Ecol 13:632–639
Hanba YT, Miyazawa S, Kogami H, Terashima I (2001) Effects of leaf age on internal CO2 transfer conductance and photosynthesis in tree species having different types of shoot phenology. Aust J Plant Physiol 28:1075–1084
Hanba YT, Kogami H, Terashima I (2002) The effect of growth irradiance on leaf anatomy and photosynthesis in Acer species differing in light adaptation. Plant Cell Environ 25:1021–1030
Hanba YT, Shibasaka M, Hayashi Y, Hayakawa T, Kasamo K, Terashima I, Katsuhara M (2004) Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants. Plant Cell Physiol 45:521–529
Harley PC, Loreto F, Marco GD, Sharkey TD (1992) Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol 98:1429–1436
Hassiotou F, Ludwig M, Renton M, Veneklaas EJ, Evans JR (2009) Influence of leaf dry mass per area, CO2, and irradiance on mesophyll conductance in sclerophylls. J Exp Bot 60:2303–2314
Haworth M, Elliott-Kingston C, McElwai JC (2011) Stomatal control as a driver of plant evolution. J Exp Bot 62:2419–2423
Kanda Y (2013) “Investigation of the freely available easy-to-use software’EZR’ for medical statistics” Bone Marrow Transplant (2013) 48:452–458
Kawase M, Hanba YT, Katsuhara M (2013) The photosynthetic response of tobacco plants overexpressing ice plant aquaporin McMIPB to a soil water deficit and high vapor pressure deficit. J Plant Res 126:517–527
Kuypers MM, Pancost RD, Damste JSS (1999) A large and abrupt fall in atmospheric CO2 concentration during Cretaceous times. Nature 399:342–345
Laisk A, Oja V, Kiirats O (1984) Assimilatory power (postillumination CO2 uptake) in leaves-measurement, environmental dependencies and kinetic properties. Plant Physiol 76:723–729
Loreto F, Harley PC, Marco GD, Sharkey TD (1992) Estimation of mesophyll conductance to CO2 flux by three different methods. Plant Physiol 98:1437–1443
Messinger SM, Buckley TN, Mott KA (2006) Evidence for involvement of photosynthetic processes in the stomatal response to CO2. Plant Physiol 140:771–778
Mizokami Y, Noguchi K, Kojima M, Sakakibara H, Terashima I (2015) Mesophyll conductance decreases in the wild type but not in an ABA-deficient mutant (aba1) of Nicotiana plumbaginifolia under drought conditions. Plant Cell Environ 38:388–398
Murakami K, Matsui R, Morimoto Y (2007) Northward invasion and range expansion of the invasive fern Thelypteris dentata (Forssk.) St. John into the urban matrix of three prefectures in Kinki District. Japan. Am Fern J 97:186–198
Nelson DD, McManus JB, Herndon SC, Zahniser MS, Tuzson B, Emmenegger L (2008) New method for isotopic ratio measurements of atmospheric carbon dioxide using a 4.3 mum pulsed quantum cascade laser. Appl Phys B 90:301–309
O’Leary MH (1981) Carbon isotope fractionation in plants. Phytochemistry 20:553–567
Ogren E, Evans JR (1993) Photosynthetic light-response curves I. The influence of CO2 partial pressure and leaf inversion. Planta 189:182–190
Pons TL, Flexas J, von Caemmerer S, Evans JR, Genty B, Ribas-Carbo M, Brugnoli E (2009) Estimating mesophyll conductance to CO2: methodology, potential errors, and recommendations. J Exp Bot 60:2217–2239
Pryer KM, Schneider H, Smith AR, Cranfill R, Wolf PG, Hunt JS, Sipes SD (2001) Horsetails and ferns are a monophyletic group and the closest living relatives to seed plants. Nature 409:618–622
Pryer KM, Schuettpelz E, Wolf PG, Schneider H, Smith AR, Cranfill R (2004) Phylogeny and evolution of ferns (monilophytes) with a focus on the early leptosporangiate divergences. Am J Bot 91:1582–1598
Roeske CA, O’Leary MH (1984) Carbon isotope effects on enzyme-catalyzed carboxylation of ribulose bisphosphate. Biochemistry 23:6275–6284
Royer DL, Berner RA, Montañez IP, Tabor NJ, Beerling DJ (2004) CO2 as a primary driver of Phanerozoic climate. GSA today 14:4–10
Sessa EB, Givnish TJ (2014) Leaf form and photosynthetic physiology of Dryopteris species distributed along light gradients in eastern North America. Funct Ecol 28:108–123
Smith AR, Pryer KM, Schuettpelz E, Korall P, Schneider H, Wolf PG (2006) A classification for extant ferns. Taxon 55:705–731
Tazoe Y, Santrucek J (2015) Superimposed behaviour of g m under ABA-induced stomata closing and low CO2. Plant Cell Environ 38:385–387
Tazoe Y, von Caemmerer S, Badger MR, Evans JR (2009) Light and CO2 do not affect the mesophyll conductance to CO2 diffusion in wheat leaves. J Exp Bot 60:2291–2301
Tazoe Y, Von Caemmerer S, Estavillo GM, Evans JR (2011) Using tunable diode laser spectroscopy to measure carbon isotope discrimination and mesophyll conductance to CO2 diffusion dynamically at different CO2 concentrations. Plant Cell Environ 34:1–12
Terashima I, Ono K (2002) Effects of HgCl2 on CO2 dependence of leaf photosynthesis: evidence indicating involvement of aquaporins in CO2 diffusion across the plasma membrane. Plant Cell Physiol 43:70–78
Terashima I, Hanba YT, Tazoe Y, Vyas P, Yano S (2006) Irradiance and phenotype: comparative eco–development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J Exp Bot 57:343–354
Terashima I, Hanba YT, Tholen D, Niinemets Ü (2011) Leaf functional anatomy in relation to photosynthesis. Plant Physiol 155:108–116
Tholen D, Ethier G, Genty B, Pepin S, Zhu X-G (2012) Variable mesophyll conductance revisited: theoretical background and experimental implications. Plant Cell Environ 35:2087–2103
Tomás M, Flexas J, Copolovici L, Galmés J, Hallik L, Medrano H, Ribas-Carbó M, Tosens T, Vislap V, Niinemets Ü (2013) Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: quantitative limitations and scaling up by models. J Exp Bot 64:2269–2281
Tuzson B, Zeeman MJ, Zahniser MS, Emmenegger L (2008) Quantum cascade laser based spectrometer for in situ stable carbon dioxide isotope measurements. Infrared Phys Technol 51:198–206
Volkova L, Tausz M, Bennett LT, Dreyer E (2009) Interactive effects of high irradiance and moderate heat on photosynthesis, pigments, and tocopherol in the tree-fern Dicksonia Antarctica. Funct Plant Biol 36:1046–1056
Vrábl D, Vasková M, Hronková M, Flexas J, Santrucek J (2009) Mesophyll conductance to CO2 transport estimated by two independent methods: effect of variable CO2 concentration and abscisic acid. J Exp Bot 60:2315–2323
Wada R, Pearce JK, Nakayama T, Matsumi Y, Hiyama T, Inoue G, Shibata T (2011) Observation of carbon and oxygen isotopic compositions of CO2 at an urban site in Nagoya using Mid-IR laser absorption spectroscopy. Atmos Environ 45:1168–1174
Woodward FI (1998) Do plants really need stomata? J Exp Bot 49:471–480
Wright IJ, Reich PB, Cornelissen JHC, Falster DS, Garnier E, Hikosaka K, Lamont BB, Lee W, Oleksyn J, Osada N, Poorter H, Villar R, Warton DI, Westoby M (2005) Assessing the generality of global leaf trait relationships. New Phytol 166:485–496
Zhang YG, Pagani M, Liu Z, Bohaty SM, DeConto R (2013) A 40–million–year history of atmospheric CO2. Philos T Roy Soc A 371:1–2
Acknowledgments
This work was supported by the New Technology Development Foundation (to N. Kodama), a Grant-in-Aid for Scientific Research for Young Researchers from the Japanese Society for the Promotion of Science (Scientific Research No. 26850009 to N. Kodama) and Collaborative Research Funding from the Solar-Terrestrial Environmental Laboratory (to N. Kodama and S. Yonemura). The authors also thank Dr. Yutaka Matsumi for giving technical advice on laser spectroscopy and Dr. Ichiro Terashima and Dr. Ko Noguchi for their insightful advice on the gas exchange system.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nishida, K., Kodama, N., Yonemura, S. et al. Rapid response of leaf photosynthesis in two fern species Pteridium aquilinum and Thelypteris dentata to changes in CO2 measured by tunable diode laser absorption spectroscopy. J Plant Res 128, 777–789 (2015). https://doi.org/10.1007/s10265-015-0736-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-015-0736-5