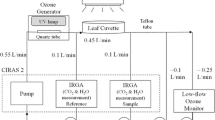
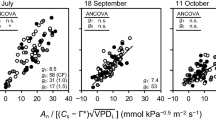
Abstract
To study the effects of different periods of ozone (O3) fumigation on photosynthesis in leaves of the Monarch birch (Betula maximowicziana), we undertook free air O3 fumigation to Monarch birch seedlings at a concentration of 60 nmol mol−1 during daytime. Plants were exposed to O3 at early, late or both periods in the growing season. The light-saturated net photosynthetic rate (A sat) in July and August was reduced by O3 exposure through a reduction in the maximum rate of carboxylation (V c,max). In early September, on the other hand, despite a reduction in V c,max, A sat was not reduced by O3 due to a counteracting increase in the stomatal conductance. Through the experiment, there was no difference in sensitivity to O3 between maturing and matured leaves. We analyzed the relationship between A sat, V c,max and accumulated stomatal O3 flux (AFst). Whereas V c,max decreased with increasing AFst, the correlation between A sat and AFst was weak because the response of stomatal conductance to O3 was affected by season. We conclude photosynthetic response of Monarch birch to O3 exposure changes with season. This is due to the inconstant stomatal response to O3 but not due to the respose of biochemical assimilation capacity in chloroplasts.


Similar content being viewed by others
References
Akimoto H (2003) Global air quality and pollution. Science 302:1716–1719
Bagard M, Le Thiec D, Delacote E, Hasenfratz-Sauder M-P, Banvoy J, Gerard J, Dizengremel P, Jolivet Y (2008) Ozone-induced changes in photosynthesis and photorespiration of hybrid poplar in relation to the developmental stage of the leaves. Physiol Plant 134:559–574
Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR, Long SP (2001) Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant, Cell Environ 24:253–259
Brendley BW, Pell EJ (1998) Ozone-induced changes in biosynthesis of Rubisco and associated compensation to stress in foliage of hybrid poplar. Tree Physiol 18:81–90
Bytnerowicz A, Godzik B, Grodzińska K, Frączek W, Musselman R, Manning W, Badea O, Popescu F, Fleischer P (2004) Ambient ozone in forests of the Central and Eastern European mountains. Environ Pollut 130:5–16
Emberson LD, Ashmore MR, Cambridge HM, Simpson D, Tuovinen JP (2000a) Modelling stomatal ozone flux across Europe. Environ Pollut 109:403–413
Emberson LD, Wieser G, Ashmore MR (2000b) Modelling stomatal conductance and ozone flux of Norway spruce: comparison with field data. Environ Pollut 109:393–402
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Günthardt-Goerg MS, Matyssek R, Scheidegger C, Keller T (1993) Differentiation and structural decline in the leaves and bark of birch (Betula pendula) under low ozone concentrations. Trees 7:104–114
Häikiö E, Freiwald V, Silfver T, Beuker E, Holopainen T, Oksanen E (2007) Impacts of elevated ozone and nitrogen on growth and photosynthesis of European aspen (Populus tremula) and hybrid aspen (P. tremula × Populus tremuloides) clones. Can J For Res 37:2326–2336
Herms DA, Mattson WJ (1992) The dilemma of plants: to grow or defend. Quart Rev Biol 67:283–335
Hokkaido Forest Tree Breeding Association (2008) Forest tree breeding and forest genetic resources in Hokkaido. Hokkaido Forest Tree Breeding Association, Ebetsu
Hoshika Y, Watanabe M, Inada N, Koike T (2012a) Modeling of stomatal conductance for estimating ozone uptake of Fagus crenata under experimentally enhanced free-air ozone exposure. Water Air Soil Pollut 223:3893–3901
Hoshika Y, Watanabe M, Inada N, Koike T (2012b) Growth and leaf gas exchange in three birch species exposed to elevated ozone and CO2 in summer. Water Air Soil Pollut 223:5017–5025
Hoshika Y, Watanabe M, Inada N, Koike T (2013a) Model-based analysis of avoidance of ozone stress by stomatal closure in Siebold’s beech (Fagus crenata). Ann Bot 112:1149–1158
Hoshika Y, Watanabe M, Inada N, Mao Q, Koike T (2013b) Photosynthetic response of early and late leaves of white birch (Betula platyphylla var. japonica) grown under free-air ozone exposure. Environ Pollut 182:242–247
Izuta T (1998) Ecophysiological response of Japanese forest tree species to ozone, simulated acid rain and soil acidification. J Plant Res 111:471–480
Jarvis PG (1976) Interpretation of variations in leaf water potential and stomatal conductance found in canopies in field. Philos Trans R Soc B 273:593–610
Kawaguchi K, Hoshika Y, Watanabe M, Koike T (2012) Ecophysiological responses of northern birch forests to the changing atmospheric CO2 and O3 concentration. Asian J Atmos Environ 6:192–205
Kikuzawa K (1983) Leaf survival of woody plants in deciduous broad-leaved forests. 1 Tall trees. Can J Bot 61:2133–2139
Kitao M, Löw M, Heerdt C, Grams TEE, Häberle K-H, Matyssek R (2009) Effects of chronic elevated ozone exposure on gas exchange responses of adult beech trees (Fagus sylvatica) as related to the within-canopy light gradient. Environ Pollut 157:537–544
Koike T (1988) Leaf structure and photosynthetic performance as related to the forest succession of deciduous broad-leaved trees. Plant Species Biol 3:77–87
Kume A, Numata S, Watanabe K, Honoki H, Nakajima H, Ishida M (2009) Influence of air pollution on the mountain forests along the Tateyama–Kurobe Alpine route. Ecol Res 24:821–830
Kurata S (1971) Illustrated important forest trees of Japan, vol 1. Chikyu Shuppan, Tokyo
Lambers H, Chapin FS III, Pons TL (2008) Plant physiological ecology, 2nd edn. Springer, New York
Long SP, Bernacchi CJ (2003) Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J Exp Bot 54:2393–2401
Mao Q, Watanabe M, Koike T (2010) Growth characteristics of two promising tree species for afforestation, birch and larch in the northeastern part of Asia. Eurasian J For Res 13:69–76
Matsuki S, Koike T (2004) Comparison of leaf life span, photosynthesis and defensive traits across seven species of deciduous broad-leaf tree seedlings. Ann Bot 97:813–817
Matsuki S, Sano Y, Koike T (2004) Chemical and physical defence in early and late leaves in three heterophyllous birch species native to northern Japan. Ann Bot 93:141–147
Matyssek R, Sandermann H (2003) Impact of ozone on trees: an ecophysiological perspective. Prog Bot 64:349–404
Matyssek R, Schnyder H, Osswald W, Ernst D, Munch JC, Pretzsch H (2012) Growth and defence in plants, Springer. Ecol St 220
Mills G, Hayes F, Wilkinson S, Davies WJ (2009) Chronic exposure to increasing background ozone impairs stomatal functioning in grassland species. Glob Chang Biol 15:1522–1533
Mills G, Pleijel H, Büker P, Braun S, Emberson LD, Harmens H, Hayes F, Simpson D, Grünhage L, Karlsson PE, Danielsson H, Bermejo V, Gonzalez-Fernandez I (2010) Mapping critical levels for vegetation. Revision undertaken in summer 2010 to include new flux-based critical levels and response functions for ozone. In: Mapping manual 2004. International Cooperative Programme on Effects of Air Pollution on Natural Vegetation and Crops
Ohara T (2011) Why is the increase of tropospheric ozone concentration in mountain and island regions in Japan? Jpn J Ecol 60:77–81 (In Japanese)
Ohno Y, Umeki K, Watanabe I, Takiya M, Terazawa K, Hara H, Matsuki S (2008) Variation in shoot mortality within crowns of severely defoliated Betula maximowicziana trees in Hokkaido, northern Japan. Ecol Res 23:355–362
Ohno Y, Umeki K, Watanabe I, Takiya M, Terazawa K, Yasaka M, Matsuki S (2009) Basal area growth and mortality of Betula maximowicziana affected by crown dieback in a secondary forest in Hokkaido, northern Japan. J For Res 14:37–43
Ohno Y, Umeki K, Terazawa K, Yasaka M, Watanabe I, Takiya M (2010) Competition as a predisposing factor of crown dieback in a secondary forest of Betula maximowicziana in Hokkaido, northern Japan. J For Res 14:37–43
Pääkkönen E, Holopainen T (1995) Influence of nitrogen supply on the response of clones of birch (Betula pendula Roth.) to ozone. New Phytol 129:595–603
Pääkkönen E, Holopainen T, Kärenlampi L (1995a) Ageing-related anatomical and ultrastructure changes in leaves of birch (Betula pendula Roth.) clones as affected by low ozone exposure. Ann Bot 75:285–294
Pääkkönen E, Metsärinne S, Holopainen T, Kärenlampi L (1995b) The ozone sensitivity of birch (Betula pendula) in relation to the developmental stage of leaves. New Phytol 132:145–154
Paoletti E, Schaub M, Matyssek R, Wieser G, Augustaitis A, Bastrup-Birk AM, Bytnerowicz A, Günthardt-Goerg MS, Müller-Starck G, Serengil Y (2010) Advances of air pollution science: from forest decline to multiple-stress effects on forest ecosystem services. Environ Pollut 158:1986–1989
Pell EJ, Sinn JP, Johansen CV (1995) Nitrogen supply as a limiting factor determining the sensitivity of Populus tremuloides Michx. to ozone stress. New Phytol 130:437–446
Peltonen PA, Vapaavuori E, Julkunen-Tiitto R (2005) Accumulation of phenolic compounds in birch leaves is changed by elevated carbon dioxide and ozone. Glob Change Biol 11:1305–1324
Polle A (1997) Defense against photooxidative damage in plants. In: Scandalios J (ed) Oxidative stress and the molecular biology of antioxidants defences. Cold Spring Harbor Laboratory Press, New York, pp 623–666
Sandermann H, Wellburn AR, Heath RL (1997) Forest decline and ozone. Springer-Verlag, Berlin
Schulze E-D, Beck E, Müller-Hohenstein K (2005) Plant ecology. Springer, Berlin/Heidelberg
Sitch S, Cox PM, Collins WJ, Huntingford C (2007) Indirect radiative forcing of climate change through ozone effects on the land-carbon sink. Nature 448:791–794
Strohm M, Eiblmeier M, Langebartels C, Jouanin L, Polle A, Sandermann H, Rennenberg H (1999) Responses of transgenic poplar (Populus tremula x P. alba) overexpressing glutathione synthetase or glutathione reductase to acute ozone stress: visible injury and leaf gas exchange. J Exp Bot 50:365–374
Tabata H (1966) A contribution to the biology of Japanese birches. Mem Coll Sci Univ Kyoto Ser B 17:239–271
Takeda M, Aihara K (2007) Effects of ambient ozone concentrations on Beech (Fagus crenanta) seedlings in the Tanzawa Mountains, Kanagawa Prefecture, Japan. J Jpn Soc Atmos Environ 42:107–117 (In Japanese with English summary)
von Caemmerer S (2000) Biochemical models of leaf photosynthesis. CSIRO publishing, Collingwood
Watanabe M, Yonekura T, Honda Y, Yoshidome M, Nakaji T, Izuta T (2005) Effects of ozone and soil water stress, singly and in combination, on leaf antioxidative systems of Fagus crenata seedlings. J Agric Meteorol 60:1105–1108
Watanabe M, Yamaguchi M, Iwasaki M, Matsuo N, Naba J, Tabe C, Matsumura H, Kohno Y, Izuta T (2006) Effects of ozone and/or nitrogen load on the growth of Larix kaempferi, Pinus densiflora and Cryptomeria japonica seedlings. J Jpn Soc Atmos Environ 41:320–334
Watanabe M, Yamaguchi M, Tabe C, Iwasaki M, Yamashita R, Funada R, Fukami M, Matsumura H, Kohno Y, Izuta T (2007) Influences of nitrogen load on the growth and photosynthetic responses of Quercus serrata seedlings to O3. Trees 21:421–432
Watanabe M, Yamaguchi M, Matsumura H, Kohno Y, Koike T, Izuta T (2012) Risk assessment of ozone impact on Fagus crenata in Japan: consideration of atmospheric nitrogen deposition. Eur J For Res 131:475–484
Watanabe M, Hoshika Y, Inada N, Wang X, Mao Q, Koike T (2013) Photosynthetic traits of Siebold’s beech and oak saplings grown under free air ozone exposure in northern Japan. Environ Pollut 174:50–56
Yamaguchi M, Watanabe M, Iwasaki M, Tabe C, Matsumura H, Kohno Y, Izuta T (2007) Growth and photosynthetic responses of Fagus crenata seedlings to O3 under different nitrogen loads. Trees 21:707–718
Yamaguchi M, Watanabe M, Matsumura H, Kohno Y, Izuta T (2011) Experimental studies on the effects of ozone on growth and photosynthetic activity of Japanese forest tree species. Asian J Atmos Environ 5:65–87
Zhang WW, Feng ZZ, Wang XK, Niu JF (2012) Responses of native broadleaved woody species to elevated ozone in subtropical China. Environ Pollut 163:149–157
Acknowledgments
This study was supported partly by the Environment Research and Technology Development Fund (5B-1105) of the Ministry of the Environment of Japan (grant to T. Koike), and by a Grant–in–Aid from the Japan Society for the Promotion of Science through its Type B program (to T. Koike, grant 23380078) and Young Scientists B (to M. Watanabe, grant 24710027 and to Y. Hoshika, grant 24780239). Thanks are also due to Dr. Anthony Garret of SCITEXT-Cambridge for English proofread.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Watanabe, M., Hoshika, Y. & Koike, T. Photosynthetic responses of Monarch birch seedlings to differing timings of free air ozone fumigation. J Plant Res 127, 339–345 (2014). https://doi.org/10.1007/s10265-013-0622-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-013-0622-y