Abstract
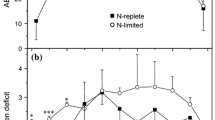
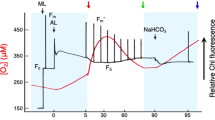
Electron transport in photosystem II (PSII) and photosystem I (PSI) was estimated in terms of chlorophyll fluorescence and changes in P700 redox, respectively, in the unicellular green alga Dunaliella salina in the presence or absence of a nitrogen source in the culture medium. In a nitrogen-containing medium, the quantum yield of PSII (ΦII) and that in PSI (ΦI) were at the same level in low light, but cyclic electron transport around photosystem I (CET-PSI) was induced under high light as estimated from an increase in ΦI/ΦII. High light might further enhance the rate of electron transport in PSI by inducing the state 2 transition, in which the distribution of light energy is shifted to PSI at the expense of PSII. Nitrogen deficiency resulted in a decrease in ΦII and an increase in ΦI. As a consequence, the rate of CET-PSI was expected to increase. The high CET-PSI under N deficiency was probably associated with a high level of energy quenching (qE) formation in PSII.




Similar content being viewed by others
References
Alric J (2010) Cyclic electron flow around photosystem I in unicellular green algae. Photosynth Res 106:54–56
Arnon D (1949) Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Asada K (1996) Radical production and scavenging in the chloroplasts. In: Baker NR (ed) Photosynthesis and the Environment. Kluwer Academic Publishers, Dordrecht, pp 128–150
Asada K (1999) The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Ben-Amotz A, Avron M (1983) On the factors which determine massive β-Carotene accumulation in the halo-tolerant alga Dunaliella bardawil. Plant Physiol 72:593–597
Bennoun P (1982) Evidence for a respiratory chain in the chloroplast. Proc Natl Acad Sci USA 79:4352–4356
Bennoun P (1994) Chlororespiration revisited: mitochondrial-plastid interactions in Chlamydomonas. Biochim Biophys Acta 1186:59–66
Bonente G, Pippa S, Castellano S, Bassi R, Ballottari M (2012) Acclimation of Chlamydomonus reinhardtii to different growth irradiances. J Biol Chem 287:5833–5847
Bukhov NG, Wiese C, Neimanis S, Heber U (1999) Heat sensitivity of chloroplasts and leaves: leakage of protons from thylakoids and reversible activation of cyclic electron transport. Photosynth Res 59:81–93
Demmig-Adams B, Adams WW III (1996) Xanthophyll cycle and light stress in nature: uniform response to excess direct sunlight among higher plant species. Planta 198:460–470
Diaz M, Haro VD, Munoz M, Quiles MJ (2007) Chlororespiration is involved in the adaptation of Brassica plants to heat and high light intensity. Plant Cell Environ 30:1578–1585
Dijkman NA, Kroon BMA (2002) Indications for chlororespiration in relation to light regime in the marine diatom Thalassiosira weissflogii. J Photochem Photobiol, B 66:179–187
Endo T, Shikanai T, Sato F, Asada K (1998) NAD(P)H dehydrogenase-dependent, antimycin A-sensitive electron donation to plastoquinone in tobacco chloroplasts. Plant Cell Physiol 39:1226–1231
Foyer C, Furbank R, Harbinson J, Horton P (1990) The mechanisms contributing to photosynthetic control of electron transport by carbon assimilation in leaves. Photosynth Res 25:83–100
Guadagno CR, De Santo AV, D’Ambrosio N (2010) A revised energy partitioning approach to assess the yields of non-photochemical quenching components. Biochim Biophys Acta 1797:525–530
Harbinson J, Foyer C (1991) Relationships between the efficiencies of photosystems I and II and stromal redox state in CO2-free air. Evidence for cyclic electron flow in vivo. Plant Physiol 97:41–49
Havaux M (1996) Short-term responses of photosystem I to heat stress. Photosynth Res 47:85–97
Heber U (2002) Irrungen, Wirrungen? The Mehler reaction in relation to cyclic electron transport in C3 plants. Photosynth Res 73:223–231
Heber U, Walker DA (1992) Concerning a dual function of coupled cyclic electron transport in leaves. Plant Physiol 100:1621–1626
Ibanez H, Ballester A, Munoz R, Quiles MJ (2010) Chlororespiration and tolerance to drought, heat and high illumination. J Plant Physiol 167:732–738
Ishida S, Morita K, Kishine M, Takabayashi A, Murakami R, Takeda S, Shimamoto K, Sato F, Endo T (2011) Allocation of absorbed light energy in Photosystem II to thermal dissipations in the presence or absence of PsbS subunits of rice. Plant Cell Physiol 52:1822–1831
Iwai M, Takizawa K, Tokutsu R, Okamuro A, Takahashi Y, Minagawa J (2010) Isolation of the elusive supercomplex that drives cyclic electron flow in photosynthesis. Nature 464:1210–1214
Jakob T, Goss R, Wilhelm C (2001) Unusual pH-dependence of diadinoxanthin de-epoxidase activation causes chlororespiratory induced accumulation of diatoxanthin in the diatom Phaeodactylum tricornutum. J Plant Physiol 158:383–390
Klughammer C, Schreiber U (1994) An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P700+-absorbance change at 830 nm. Planta 192:261–268
Makino A, Miyake C, Yokota A (2002) Physiological functions of the water–water cycle (Mehler reaction) and the cyclic electron flow around PSI in rice leaves. Plant Cell Physiol 43:1017–1026
Mi H, Endo T, Ogawa T, Asada K (1995) Thylakoid membrane-bound NADPH-specific pyridine nucleotide dehydrogenase complex mediates cyclic electron transport in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol 36:661–668
Miyake C (2010) Alternative electron flows (water–water cycle and cyclic electron flow around PSI) in photosynthesis: molecular mechanisms and physiological functions. Plant Cell Physiol 51:1951–1963
Miyake C, Shinzaki Y, Miyata M, Tomizawa K (2004) Enhancement of cyclic electron flow around PSI at high light and its contribution to the induction of non-photochemical quenching of Chl fluorescence in intact leaves of tobacco plants. Plant Cell Physiol 45:1433–1462
Miyake C, Miyata M, Shinzaki Y, Tomizawa K (2005a) CO2 response of cyclic electron flow around PSI (CEF-PSI) in tobacco leaves—relative electron fluxes through PSI and PSII determine the magnitude of non-photochemical quenching (NPQ) of Chl fluorescence. Plant Cell Physiol 46:629–637
Miyake C, Horiguchi S, Makino A, Shinzaki Y, Yamamoto H, Tomizawa K (2005b) Effects of light intensity on cyclic electron flow around PSI and its relationship to non-photochemical quenching of Chl fluorescence in tobacco leaves. Plant Cell Physiol 46:1819–1830
Müller P, Li X-P, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566
Munekage Y, Hashimoto M, Miyake C, Tomizawa KI, Endo T, Tasaka M, Shikanai T (2004) Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 429:579–582
Niyogi KK (2000) Safety valves for photosynthesis. Curr Opin Plant Biol 3:455–460
Niyogi KK, Grossman AR, Björkman O (1998) Arabidopsis mutants define a central role for the xanthophylls cycle in the regulation of photosynthetic energy conversion. Plant Cell 10:1121–1134
Peltier G, Tolleter D, Billon E, Cournac L (2010) Auxiliary electron transport pathways in chloroplasts of microalgae. Photosynth Res 106:19–31
Quick WP, Stitt M (1989) An examination of factors contributing to non-photochemical quenching of chlorophyll fluorescence in barley leaves. Biochim Biophys Acta 977:287–296
Quiles MJ (2006) Stimulation of chlororespiration by heat and high light intensity in oat plants. Plant Cell Environ 29:1463–1470
Scheibe R, Stitt M (1988) Comparison of NADP-malate dehydrogenase activation, QA reduction and 02 evolution in spinach leaves. Plant Physiol Biochem 26:473–482
Schoen M (1988) Cell counting. In: Lobban C, Champan D, Kermer BP (eds) Experimental Phycology. Cambridge University Press, Cambridge
Schreiber U, Schliwa U, Bilger W (1986) Continuous recording of photochemical and non-photochemical chlorophyll quenching with a new type of modulation fluorometer. Photosynth Res 10:51–62
Schreiber U, Endo T, Mi H, Asada K (1995) Quenching analysis of chlorophyll fluorescence by a saturation pulse method: particular aspects relating to the study of eukaryotic algae and cyanobacteria. Plant Cell Physiol 36:873–882
Shariati M, Lilley MC (1994) Loss of intracellular glycerol from Dunaliella by electroporation at constant osmotic pressure: subsequent restoration of glycerol content and associated volume changes. Plant Cell Environ 17:1295–1304
Ting CS, Owens TG (1993) Photochemical and nonphotochemical fluorescence quenching processes in the diatom Phaeodactylum tricornutum. Plant Physiol 101:1323–1330
Acknowledgments
This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and from the Ministry of Agriculture and Fishery of Japan to T. E. We would also like to thank the Iranian Ministry of Science, Research and Technology, and Office of Graduate Studies, University of Isfahan, for financial support to A. E. while visiting Japan on sabbatical.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Einali, A., Shariati, M., Sato, F. et al. Cyclic electron transport around photosystem I and its relationship to non-photochemical quenching in the unicellular green alga Dunaliella salina under nitrogen deficiency. J Plant Res 126, 179–186 (2013). https://doi.org/10.1007/s10265-012-0512-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-012-0512-8