Abstract
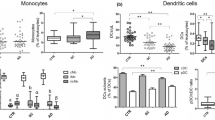
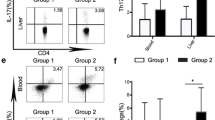
Primary biliary cirrhosis (PBC) is a progressive autoimmune liver disease in which monocytes/macrophages infiltration and skewed T helper type (Th) 1 and Th17 cell responses participate in the development of the disease. Human peripheral blood monocytes are heterogeneous and can be divided into classical CD14highCD16−, intermediate CD14highCD16+, and nonclassical CD14lowCD16+ monocyte subsets. Compared to classical monocytes, CD16+ monocytes are generally termed pro-inflammatory monocytes and play an important pathogenic role in autoimmune diseases. However, little is known about the immunophenotype and immunopathogenic role of peripheral blood CD16+ monocytes in PBC. Thus, we investigated the phenotype and function of these circulating monocyte subsets from PBC patients. The frequencies of circulating CD14highCD16+ and CD14lowCD16+ subpopulation were increased in disease compared with healthy controls. Among them, CD14lowCD16+ monocyte subset positively correlated with disease progress, liver damage indicators and serum C-reactive protein, respectively. Furthermore, the frequencies of Th1 and Th17 cells were upregulated and CD14lowCD16+ monocyte subset was also positively associated with Th1 cell frequency in PBC. Using a vitro coculture model, we further found that CD14lowCD16+ monocytes promoted Th1 cell polarization compared to classical monocytes. Interleukin-12 (IL-12) and direct contact of patient CD4+T cell and CD14lowCD16+ monocytes, were responsible for CD14lowCD16+ monocytes promotion of Th1 cells polarization in PBC. Our study demonstrated that the enhanced CD14lowCD16+ monocyte subset participated in fostering liver damage and inflammatory responses, and promoted Th1 cells skewing in PBC.




Similar content being viewed by others
Abbreviations
- PBC:
-
Primary biliary cirrhosis
- ADA:
-
Adenosine deaminase
- AST:
-
Aspartate aminotransferase
- ALT:
-
Alanine aminotransferase
- ALP:
-
Alkaline phosphatase
- GGT:
-
γ-Glutamyltranspeptidase
- TBIL:
-
Total bilirubin
- CRP:
-
C-reactive protein
- Th:
-
T helper type
- RA:
-
Rheumatoid arthritis
- ITP:
-
Idiopathic thrombocytopenic purpura
- CHB:
-
Chronic hepatitis B
- HC:
-
Healthy control
- AMA:
-
Anti-mitochondrial antibodies
- PBMCs:
-
Peripheral blood mononuclear cells
- IL-12:
-
Interleukin-12
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
References
Ziegler-Heitbrock L, Ancuta P, Crowe S, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–80.
Ziegler-Heitbrock L. The CD14 + CD16 + blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–92.
Wong KL, Tai JJ, Wong WC, et al. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118:e16–31.
Belge KU, Dayyani F, Horelt A, et al. The proinflammatory CD14 + CD16 + DR ++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–42.
Zawada AM, Rogacev KS, Rotter B, et al. SuperSAGE evidence for CD14 ++CD16 + monocytes as a third monocyte subset. Blood. 2011;118:e50–61.
Anbazhagan K, Duroux-Richard I, Jorgensen C, et al. Transcriptomic network support distinct roles of classical and non-classical monocytes in human. Int Rev Immunol. 2014;33:470–89.
Seidler S, Zimmermann HW, Weiskirchen R, et al. Elevated circulating soluble interleukin-2 receptor in patients with chronic liver diseases is associated with non-classical monocytes. BMC Gastroenterol. 2012;12:38.
Fingerle G, Pforte A, Passlick B, et al. The novel subset of CD14 +/CD16 + blood monocytes is expanded in sepsis patients. Blood. 1993;82:3170–6.
Shalova IN, Kajiji T, Lim JY, et al. CD16 regulates TRIF-dependent TLR4 response in human monocytes and their subsets. J Immunol. 2012;188:3584–93.
Ancuta P, Kunstman KJ, Autissier P, et al. CD16 + monocytes exposed to HIV promote highly efficient viral replication upon differentiation into macrophages and interaction with T cells. Virology. 2006;344:267–76.
Williams DW, Byrd D, Rubin LH, et al. CCR2 on CD14(+)CD16(+) monocytes is a biomarker of HIV-associated neurocognitive disorders. Neurol Neuroimmunol Neuroinflamm. 2014;1:e36.
Rossol M, Kraus S, Pierer M, et al. The CD14(bright) CD16 + monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum. 2012;64:671–7.
Zhong H, Bao W, Li X, et al. CD16 + monocytes control T-cell subset development in immune thrombocytopenia. Blood. 2012;120:3326–35.
Grip O, Bredberg A, Lindgren S, et al. Increased subpopulations of CD16(+) and CD56(+) blood monocytes in patients with active Crohn’s disease. Inflamm Bowel Dis. 2007;13:566–72.
Selmi C, Lleo A, Pasini S, et al. Innate immunity and primary biliary cirrhosis. Curr Mol Med. 2009;9:45–51.
Harada K, Shimoda S, Sato Y, et al. Periductal interleukin-17 production in association with biliary innate immunity contributes to the pathogenesis of cholangiopathy in primary biliary cirrhosis. Clin Exp Immunol. 2009;157:261–70.
Yang CY, Ma X, Tsuneyama K, et al. IL-12/Th1 and IL-23/Th17 biliary microenvironment in primary biliary cirrhosis: implications for therapy. Hepatology. 2014;59:1944–53.
Rong G, Zhou Y, Xiong Y, et al. Imbalance between T helper type 17 and T regulatory cells in patients with primary biliary cirrhosis: the serum cytokine profile and peripheral cell population. Clin Exp Immunol. 2009;156:217–25.
Serbina NV, Jia T, Hohl TM, et al. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–52.
Evans HG, Gullick NJ, Kelly S, et al. In vivo activated monocytes from the site of inflammation in humans specifically promote Th17 responses. Proc Natl Acad Sci USA. 2009;106:6232–7.
Zhang JY, Zou ZS, Huang A, et al. Hyper-activated pro-inflammatory CD16 monocytes correlate with the severity of liver injury and fibrosis in patients with chronic hepatitis B. PLoS ONE. 2011;6:e17484.
Allina J, Stanca CM, Garber J, et al. Anti-CD16 autoantibodies and delayed phagocytosis of apoptotic cells in primary biliary cirrhosis. J Autoimmun. 2008;30:238–45.
Zimmermann HW, Seidler S, Nattermann J, et al. Functional contribution of elevated circulating and hepatic non-classical CD14CD16 monocytes to inflammation and human liver fibrosis. PLoS ONE. 2010;5:e11049.
Blom L, Poulsen LK. IL-1 family members IL-18 and IL-33 upregulate the inflammatory potential of differentiated human Th1 and Th2 cultures. J Immunol. 2012;189:4331–7.
Ikeda A, Aoki N, Kido M, et al. Progression of autoimmune hepatitis is mediated by IL-18-producing dendritic cells and hepatic CXCL9 expression in mice. Hepatology. 2014;60:224–36.
Fernandez E, Rodrigo L, Riestra S, et al. Adenosine deaminase isoenzymes and neopterin in liver cirrhosis. J Clin Gastroenterol. 2000;30:181–6.
Kobayashi F, Ikeda T, Marumo F, et al. Adenosine deaminase isoenzymes in liver disease. Am J Gastroenterol. 1993;88:266–71.
Sanchez RA, Hueso PJ, Rico SJ, et al. Enzymatic activity of serum adenosine deaminase in different liver disorders. An Med Interna. 1989;6:300–4.
Nilius R, Neef L, Rath FW, et al. Adenosine deaminase activity—an indicator of inflammation in liver disease. Dtsch Z Verdau Stoffwechselkr. 1987;47:224–9.
Alempijevic T, Krstic M, Jesic R, et al. Biochemical markers for non-invasive assessment of disease stage in patients with primary biliary cirrhosis. World J Gastroenterol. 2009;15:591–4.
Sapey T, Mendler MH, Guyader D, et al. Respective value of alkaline phosphatase, gamma-glutamyl transpeptidase and 5′ nucleotidase serum activity in the diagnosis of cholestasis: a prospective study of 80 patients. J Clin Gastroenterol. 2000;30:259–63.
Suzuki N, Irie M, Iwata K, et al. Altered expression of alkaline phosphatase (ALP) in the liver of primary biliary cirrhosis (PBC) patients. Hepatol Res. 2006;35:37–44.
Lammers WJ, van Buuren HR, Hirschfield GM, et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology. 2014;147(1338–1349):e15.
Lammers WJ, Kowdley KV, van Buuren HR, et al. Predicting outcome in primary biliary cirrhosis. Ann Hepatol. 2014;13:316–26.
Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–12.
Wong KL, Yeap WH, Tai JJ, et al. The three human monocyte subsets: implications for health and disease. Immunol Res. 2012;53:41–57.
Evans HG, Suddason T, Jackson I, et al. Optimal induction of T helper 17 cells in humans requires T cell receptor ligation in the context of Toll-like receptor-activated monocytes. Proc Natl Acad Sci USA. 2007;104:17034–9.
Acknowledgments
This work was supported by the National Natural Science Foundation project of China (81303287) and funded by Guangdong Province Medical Research Foundation project (A2013236).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest in connection with this study.
Additional information
Anping Peng and Peifeng Ke have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Peng, A., Ke, P., Zhao, R. et al. Elevated circulating CD14lowCD16+ monocyte subset in primary biliary cirrhosis correlates with liver injury and promotes Th1 polarization. Clin Exp Med 16, 511–521 (2016). https://doi.org/10.1007/s10238-015-0381-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-015-0381-2