Abstract
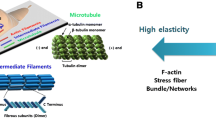
The understanding of the self-regulation of the mechanical properties in non-sarcomeric cells, such as lung cells or cells during tissue development, remains an open research problem with many unresolved issues. Their behaviour is far from the image of the traditionally studied sarcomeric cells, since the crosstalk between the signalling pathways and the complexity of the mechanical properties creates an intriguing mechano-chemical coupling. In these situations, the inelastic effects dominate the cytoskeletal structure showing phenomena like fluidisation and subsequent solidification. Here, we proposes the inelastic contractile unit framework as an attempt to reconciles these effects. The model comprises a mechanical description of the nonlinear elasticity of the cytoskeleton incorporated into a continuum-mechanics framework using the eighth-chains model. In order to address the inelastic effect, we incorporate the dynamic of crosslinks, considering the \(\alpha \)-actinin and the active stress induced by the myosin molecular motors. Finally, we introduce a hypothesis that links the ability to fluidise and re-solidify as a consequence of the interaction between the active stress and the gelation state defined by the crosslinks. We validate the model with data obtained from experiments of drug-induced relaxation reported in the literature.








Similar content being viewed by others
References
An S, Fabry B, Trepat X, Wang N, Fredberg J (2006) Do biophysical properties of the airway smooth muscle in culture predict airway hyperresponsiveness? Am J Respir Cell Mol Biol 35(1):55–64
Bausch AR, Kroy K (2006) A bottom-up approach to cell mechanics. Nat Phys 2(4):231–238
Bendix P, Koenderink G, Cuvelier D, Dogic Z, Koeleman BN, Brieher W, Field C, Mahadevan L, Weitz D (2008) A quantitative analysis of contractility in active cytoskeletal protein networks. Biophys J 94(8):36–3126
Besser A, Schwarz U (2007) Coupling biochemistry and mechanics in cell adhesion: a model for inhomogeneous stress fiber contraction. N J Phys 9(11):425–425
Brown A, Litvinov R, Discher D, Purohit P, Weisel J (2009) Multiscale mechanics of fibrin polymer: gel stretching with protein unfolding and loss of water. Science 325(5941):4–741
Cipelletti L, Ramos L (2005) Slow dynamics in glassy soft matter. J Phys Condens Matter 17(6):253–285
Colombelli J, Besser A, Kress H, Reynaud E, Girard P, Caussinus E, Haselmann U, Small J, Schwarz U, Stelzer E (2009) Mechanosensing in actin stress fibers revealed by a close correlation between force and protein localization. J Cell Sci 122(Pt 10):79–1665
De Gennes P-G (1979) Scaling concepts in polymer physics. Cornell University Press, Ithaca
Ehrlicher AJ, Krishnan R, Guo M, Bidan CM, Weitz D, Pollak M (2015) Alpha-actinin binding kinetics modulate cellular dynamics and force generation. PNAS 112(21):6619–6624
Fabry B, Maksym GN, Butler J, Glogauer M, Navajas D, Fredberg J (2001) Scaling the microrheology of living cells. Phys Rev Lett 87(14):148102
Fabry B, Maksym GN, Butler J, Glogauer M, Navajas D, Taback N, Millet E, Fredberg J (2003) Time scale and other invariants of integrative mechanical behavior in living cells. Phys Rev E 68(4):041914
Gardel M, Shin J, Mackintosh F, Mahadevan L, Matsudaira P, Weitz D (2004) Elastic behavior of cross-linked and bundled actin networks. Science 304(5675):5–1301
Golji J, Collins R, Mofrad M (2009) Molecular mechanics of the \(\alpha \)-actinin rod domain: bending, torsional, and extensional behavior. PLoS Comput Biol 5:e1000389
Gunst S, Fredberg J (2003) The first three minutes: smooth muscle contraction, cytoskeletal events, and soft glasses. J Appl Physiol 95(1):413–425
Gunst S, Zhang W (2008) Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol 295(3):C576–C587
Hirshman C, Zhu D, Panettieri R, Emala C (2001) Actin depolymerization via the beta-adrenoceptor in airway smooth muscle cells: a novel PKA-independent pathway. Am J Physiol Cell Physiol 281(5):C1468–C1476
Hirshman C, Zhu D, Pertel T, Panettieri R, Emala C (2005) Isoproterenol induces actin depolymerization in human airway smooth muscle cells via activation of an Src kinase and GS. Am J Physiol Lung Cell Mol Physiol 288(5):L924–L931
Joanny J, Prost J (2009) Active gels as a description of the actin–myosin cytoskeleton. HFSP J 3(2):94–104
Kassianidou E, Kumar S (2015) A biomechanical perspective on stress fiber structure and function. Biochim Biophys Acta Mol Cell Res 1853(11):3065–3074
Kim T, Hwang W, Lee H, Kamm R (2009) Computational analysis of viscoelastic properties of crosslinked actin networks. PLoS Comput Biol 5(7):e1000439
Lieleg O, Claessens M, Heussinger C, Frey E, Bausch A (2007) Mechanics of bundled semiflexible polymer networks. Phys Rev Lett 99(8):088102
Lieleg O, Kayser J, Brambilla G, Cipelletti L, Bausch A (2011) Slow dynamics and internal stress relaxation in bundled cytoskeletal networks. Nat Mater 10(3):236–242
Liu J, Taylor DW, Taylor K (2004) A 3-D reconstruction of smooth muscle alpha-actinin by CryoEm reveals two different conformations at the actin-binding region. J Mol Biol 338(1):25–115
Liverpool T (2006) Active gels: where polymer physics meets cytoskeletal dynamics. Philos Trans R Soc A 364(1849):55–3335
López-Menéndez H, Rodríguez J (2016) Microstructural model for cyclic hardening in f-actin networks crosslinked by \(\alpha \)-actinin. J Mech Phys Solids 91:28–39
Mackintosh F, Kas J, Janmey P (1995) Elasticity of semiflexible biopolymer networks. Phys Rev Lett 75:4425
Mandadapu K, Govindjee S, Mofrad M (2008) On the cytoskeleton and soft glassy rheology. J Biomech 41(7):1467–1478
Mizuno D, Tardin C, Schmidt CF, Mackintosh FC (2008) Nonequilibrium mechanics of active cytoskeletal networks. Science 315(5810):370–373
Murrell M, Gardel M (2012) F-actin buckling coordinates contractility and severing in a biomimetic actomyosin cortex. PNAS 109(51):20820–20825
Murtada S, Kroon M, Holzapfel G (2010) A calcium-driven mechanochemical model for prediction of force generation in smooth muscle. Biomech Model Mechanobiol 9(6):62–749
Palmer J, Boyce M (2008) Constitutive modeling of the stress–strain behavior of F-actin filament networks. Acta Biomater 4(3):597–612
Purohit P, Litvinov R, Brown A, Discher D, Weisel J (2011) Protein unfolding accounts for the unusual mechanical behavior of fibrin networks. Acta Biomater 7(6):2374–2383
Schmoller K, Lieleg O, Bausch A (2009) Structural and viscoelastic properties of actin/filamin networks: cross-linked versus bundled networks. Biophys J 97(1):9–83
Schmoller K, Fernandez P, Arevalo R, Blair D, Bausch A (2010) Cyclic hardening in bundled actin networks. Nat Commun 1:134
Sollich P, Lequeux F, Hébraud P, Cates ME (1997) Rheology of soft glassy materials. Phys Rev Lett 78(10):2020
Stamenović D, Suki B, Fabry B, Wang N, Fredberg J, Buy J (2004) Rheology of airway smooth muscle cells is associated with cytoskeletal contractile stress. J Appl Physiol 96(5):1600–1605
Trepat X, Deng L, An S, Navajas D, Tschumperlin D, Gerthoffer W, Butler J, Fredberg J (2007) Universal physical responses to stretch in the living cell. Nature 447(7144):592–595
Vigliotti A, Ronan R, Baaijens FPT, Deshpande VS (2016) A thermodynamically motivated model for stress-fiber reorganization. Biomech Model Mechanobiol 15(4):761–789
Wagner B, Tharmann R, Haase I, Fischer M, Bausch A (2006) Cytoskeletal polymer networks: the molecular structure of cross-linkers determines macroscopic properties. PNAS 103(38):8–13974
Wolff L, Fernandez P, Kroy K (2010) Inelastic mechanics of sticky biopolymer networks. N J Phys 12(5):053024
Wolff L, Fernandez P, Kroy K (2012) Resolving the stiffening–softening paradox in cell mechanics. PloS ONE 7(7):e40063
Yao N, Broedersz C, Depken M, Becker D, Pollak M, Mackintosh F, Weitz D (2013) Stress-enhanced gelation: a dynamic nonlinearity of elasticity. Phys Rev Lett 110(1):018103
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
López-Menéndez, H., Rodríguez, J.F. Towards the understanding of cytoskeleton fluidisation–solidification regulation. Biomech Model Mechanobiol 16, 1159–1169 (2017). https://doi.org/10.1007/s10237-017-0878-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-017-0878-6