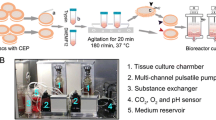
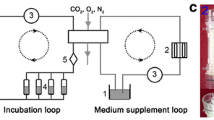
Abstract
Evidence suggests that mechanical load is related to structural destruction of disk annulus fibrosus (AF) either in adult disk degeneration or in child disk acute injury. Both biochemical and biomechanical properties are different between immature and mature disks. However, the effects of mechanical compression on immature AF are not fully clear. This study was to investigate the effects of a relatively wide range of dynamic compressive frequency on matrix homeostasis within the immature AF. Immature disks from pig (3–4 months) were randomly assigned into the control group (non-compression) and compression groups (0.1, 0.5, 1.0, 3.0 and 5.0 Hz). All disks were bioreactor-cultured for 7 days. AF matrix production was evaluated by histology, gene expression, glycosaminoglycan (GAG) content, hydroxyproline (HYP) content and immunohistochemistry. Generally, no obvious difference was found in HE staining between control group and compression groups. However, alcian blue staining indicated proteoglycan content in the 5.0-Hz group was decreased compared with the control group and other compression groups. Similarly, a catabolic remodeling gene expression profile with the down-regulated matrix genes (aggrecan, collagen I and collagen II) and tissue inhibitor of metalloproteinases (TIMP-1 and TIMP-3) and the up-regulated matrix catabolic enzymes (ADAMTS-4 and MMP-3) was found in the 5.0-Hz group. Further analysis indicated that GAG content, HYP content and aggrecan protein deposition were also decreased in the 5.0-Hz group. Hence, we concluded that matrix homeostasis within the immature AF was compressive frequency dependent, and the relatively higher frequency (5.0 Hz) is unfavorable for matrix production within the immature AF. These findings will contribute to further understanding of the relationship between mechanical compression and immature AF biosynthesis.








Similar content being viewed by others
References
Alini M et al (2008) Are animal models useful for studying human disc disorders/degeneration? Euro Spine J 17:2–19. doi:10.1007/s00586-007-0414-y
Bach FC, de Vries SA, Krouwels A, Creemers LB, Ito K, Meij BP, Tryfonidou MA (2015) The species-specific regenerative effects of notochordal cell-conditioned medium on chondrocyte-like cells derived from degenerated human intervertebral discs. Eur Cells & Mater 30:132–146 discussion 146–137
Baer AE, Laursen TA, Guilak F, Setton LA (2003) The micromechanical environment of intervertebral disc cells determined by a finite deformation, anisotropic, and biphasic finite element model. J Biomech Eng 125:1–11
Beckstein JC, Sen S, Schaer TP, Vresilovic EJ, Elliott DM (2008) Comparison of animal discs used in disc research to human lumbar disc: axial compression mechanics and glycosaminoglycan content. Spine 33:E166–173. doi:10.1097/BRS.0b013e318166e001
Buschmann MD, Hunziker EB, Kim YJ, Grodzinsky AJ (1996) Altered aggrecan synthesis correlates with cell and nucleus structure in statically compressed cartilage. J Cell Sci 109(Pt 2):499–508
Cho H, Park SH, Lee S, Kang M, Hasty KA, Kim SJ (2011) Snapshot of degenerative aging of porcine intervertebral disc: a model to unravel the molecular mechanisms. Exp Mol Med 43:334–340. doi:10.3858/emm.2011.43.6.036
Cho H, Lee S, Park SH, Huang J, Hasty KA, Kim SJ (2013a) Synergistic effect of combined growth factors in porcine intervertebral disc degeneration. Connect Tissue Res 54:181–186. doi:10.3109/03008207.2013.775258
Cho H et al (2013b) Construction of a tissue-engineered annulus fibrosus. Artif Org 37:E131–138. doi:10.1111/aor.12066
Cho H, Holt DC 3rd, Smith R, Kim SJ, Gardocki RJ, Hasty KA (2015) The effects of platelet-rich plasma on halting the progression in porcine intervertebral disc degeneration. Artif Org. doi:10.1111/aor.12530
Dang L, Liu Z (2010) A review of current treatment for lumbar disc herniation in children and adolescents. Eur Spine J 19:205–214. doi:10.1007/s00586-009-1202-7
Elliott DM, Sarver JJ (2004) Young investigator award winner: validation of the mouse and rat disc as mechanical models of the human lumbar disc. Spine 29:713–722 (Phila Pa 1976)
Farndale RW, Sayers CA, Barrett AJ (1982) A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res 9:247–248
Gennuso R, Humphreys RP, Hoffman HJ, Hendrick EB, Drake JM (1992) Lumbar intervertebral disc disease in the pediatric population. Pediatr Neurosurg 18:282–286
Guilak F, Ratcliffe A, Mow VC (1995) Chondrocyte deformation and local tissue strain in articular cartilage: a confocal microscopy study. J Orthop Res 13:410–421. doi:10.1002/jor.1100130315
Guterl CC et al (2013) Challenges and strategies in the repair of ruptured annulus fibrosus. Eur Cell Mater 25:1–21
Haidar R, Ghanem I, Saad S, Uthman I (2010) Lumbar disc herniation in young children. Acta Paediatr 99:19–23. doi:10.1111/j.1651-2227.2009.01460.x
Haschtmann D, Stoyanov JV, Ettinger L, Nolte LP, Ferguson SJ (2006) Establishment of a novel intervertebral disc/endplate culture model: analysis of an ex vivo in vitro whole-organ rabbit culture system. Spine 31:2918–2925. doi:10.1097/01.brs.0000247954.69438.ae (Phila Pa 1976)
Hee HT, Zhang J, Wong HK (2010) An in vitro study of dynamic cyclic compressive stress on human inner annulus fibrosus and nucleus pulposus cells. Spine J 10:795–801. doi:10.1016/j.spinee.2010.06.009
Hsieh AH, Twomey JD (2010) Cellular mechanobiology of the intervertebral disc: new directions and approaches. J Biomech 43:137–145. doi:10.1016/j.jbiomech.2009.09.019
Hunter CJ, Matyas JR, Duncan NA (2003) The three-dimensional architecture of the notochordal nucleus pulposus: novel observations on cell structures in the canine intervertebral disc. J Anat 202:279–291
Illien-Junger S, Gantenbein-Ritter B, Grad S, Lezuo P, Ferguson SJ, Alini M, Ito K (2010) The combined effects of limited nutrition and high-frequency loading on intervertebral discs with endplates. Spine 35:1744–1752. doi:10.1097/BRS.0b013e3181c48019 (Phila Pa 1976)
Kasra M, Goel V, Martin J, Wang ST, Choi W, Buckwalter J (2003) Effect of dynamic hydrostatic pressure on rabbit intervertebral disc cells. J Orthop Res 21:597–603. doi:10.1016/S0736-0266(03)00027-5
Kasra M, Merryman WD, Loveless KN, Goel VK, Martin JD, Buckwalter JA (2006) Frequency response of pig intervertebral disc cells subjected to dynamic hydrostatic pressure. J Orthop Res 24:1967–1973. doi:10.1002/jor.20253
Korecki CL, MacLean JJ, Iatridis JC (2007) Characterization of an in vitro intervertebral disc organ culture system. Eur Spine J 16:1029–1037. doi:10.1007/s00586-007-0327-9
Korecki CL, Kuo CK, Tuan RS, Iatridis JC (2009) Intervertebral disc cell response to dynamic compression is age and frequency dependent. J Orthop Res 27:800–806. doi:10.1002/jor.20814
Kurihara A, Kataoka O (1980) Lumbar disc herniation in children and adolescents. A review of 70 operated cases and their minimum 5-year follow-up studies. Spine 5:443–451 (Phila Pa 1976)
Le Maitre CL, Freemont AJ, Hoyland JA (2005) The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther 7:R732–745. doi:10.1186/ar1732
Li ST et al (2014) A novel axial-stress bioreactor system combined with a substance exchanger for tissue engineering of 3D constructs tissue engineering part C. Methods 20:205–214. doi:10.1089/ten.TEC.2013.0173
Lundin O, Ekstrom L, Hellstrom M, Holm S, Sward L (2000) Exposure of the porcine spine to mechanical compression: differences in injury pattern between adolescents and adults. Eur Spine J 9:466–471
MacGee EE (1968) Protruded lumbar disc in a 9-year-old boy. J Pediatr 73:418–419
Maclean JJ, Lee CR, Alini M, Iatridis JC (2004) Anabolic and catabolic mRNA levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. J Orthop Res 22:1193–1200. doi:10.1016/j.orthres.2004.04.004
Ohshima H, Urban JP (1992) The effect of lactate and pH on proteoglycan and protein synthesis rates in the intervertebral disc. Spine 17:1079–1082 (Phila Pa 1976)
Ohshima H, Tsuji H, Hirano N, Ishihara H, Katoh Y, Yamada H (1989) Water diffusion pathway, swelling pressure, and biomechanical properties of the intervertebral disc during compression load. Spine 14:1234–1244 (Phila Pa 1976)
Palmer EI, Lotz JC (2004) The compressive creep properties of normal and degenerated murine intervertebral discs. J Orthop Res 22:164–169. doi:10.1016/S0736-0266(03)00161-X
Pope MH, Magnusson M, Wilder DG (1998) Kappa Delta Award. Low back pain and whole body vibration. Clin Orthop Relat Res 354:241–248
Setton LA, Chen J (2004) Cell mechanics and mechanobiology in the intervertebral disc. Spine 29:2710–2723
Sharifi S, Bulstra SK, Grijpma DW, Kuijer R (2015) Treatment of the degenerated intervertebral disc; closure, repair and regeneration of the annulus fibrosus. J Tissue Eng Regen Med 9:1120–1132. doi:10.1002/term.1866
Stokes IA, Iatridis JC (2004) Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. Spine 29:2724–2732 (Phila Pa 1976)
Walsh AJ, Lotz JC (2004) Biological response of the intervertebral disc to dynamic loading. J Biomech 37:329–337
Wilder DG, Pope MH (1996) Epidemiological and aetiological aspects of low back pain in vibration environments - an update. Clinical biomechanics (Bristol, Avon) 11:61–73
Acknowledgments
We gratefully acknowledge the funding from the National Natural Science Foundation of China (NSFC 81272029 and NSFC 81027005) and Science and Technology Achievement Transformation Fund of the Third Military Medical University (2011XZH006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None of the authors has any conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Li, P., Gan, Y., Xu, Y. et al. Matrix homeostasis within the immature annulus fibrosus depends on the frequency of dynamic compression: a study based on the self-developed mechanically active bioreactor. Biomech Model Mechanobiol 16, 385–394 (2017). https://doi.org/10.1007/s10237-016-0823-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-016-0823-0