Abstract
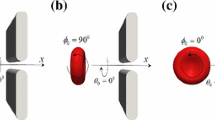
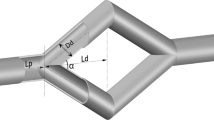
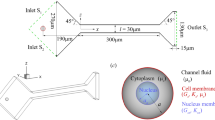
The narrow slit between endothelial cells that line the microvessel wall is the principal pathway for tumor cell extravasation to the surrounding tissue. To understand this crucial step for tumor hematogenous metastasis, we used dissipative particle dynamics method to investigate an individual cell passing through a narrow slit numerically. The cell membrane was simulated by a spring-based network model which can separate the internal cytoplasm and surrounding fluid. The effects of the cell elasticity, cell shape, nucleus and slit size on the cell transmigration through the slit were investigated. Under a fixed driving force, the cell with higher elasticity can be elongated more and pass faster through the slit. When the slit width decreases to 2/3 of the cell diameter, the spherical cell becomes jammed despite reducing its elasticity modulus by 10 times. However, transforming the cell from a spherical to ellipsoidal shape and increasing the cell surface area by merely 9.3 % can enable the cell to pass through the narrow slit. Therefore, the cell shape and surface area increase play a more important role than the cell elasticity in cell passing through the narrow slit. In addition, the simulation results indicate that the cell migration velocity decreases during entrance but increases during exit of the slit, which is qualitatively in agreement with the experimental observation.












Similar content being viewed by others
References
Albelda SM, Smith CW, Ward PA (1994) Adhesion molecules and inflammatory injury. Faseb J 8:504–512
Boey SK, Boal DH, Discher DE (1998) Simulations of the erythrocyte cytoskeleton at large deformation. I Microsc Models Biophys J 75:1573–1583
Chaw KC, Manimaran M, Tay EH, Swaminathan S (2007) Multi-step microfluidic device for studying cancer metastasis. Lab Chip 7:1041–1047
Chaw KC, Manimaran M, Tay FEH, Swaminathan S (2006) A quantitative observation and imaging of single tumor cell migration and deformation using a multi-gap microfluidic device representing the blood vessel. Microvasc Res 72:153–160
Chen MB, Whisler JA, Jeon JS, Kamm RD (2013) Mechanisms of tumor cell extravasation in an in vitro microvascular network platform. Integr Biol UK 5:1262–1271
Cross SE, Jin YS, Rao J, Gimzewski JK (2007) Nanomechanical analysis of cells from cancer patients. Nat Nanotechnol 2:780–783
Dao M, Li J, Suresh S (2006) Molecularly based analysis of deformation of spectrin network and human erythrocyte. Mat Sci Eng C Bio S 26:1232–1244
Dewitt S, Hallett M (2007) Leukocyte membrane “expansion”: a central mechanism for leukocyte extravasation. J Leukoc Biol 81:1160–1164
Espanol P (1995) Hydrodynamics from dissipative particle dynamics. Phys Rev E 52:1734–1742
Fan J, Fu BM (2015) Quantification of malignant breast cancer cell MDA-MB-231 transmigration across brain and lung microvascular endothelium. Ann Biomed Eng. doi:10.1007/s10439-015-1517-y
Fan XJ, Phan-Thien N, Chen S, Wu XH, Ng TY (2006) Simulating flow of DNA suspension using dissipative particle dynamics. Phys Fluids 18(6):063102
Fedosov DA, Caswell B, Karniadakis GE (2010) Systematic coarse-graining of spectrin-level red blood cell models. Comput Method Appl M 199:1937–1948
Fedosov DA, Gompper G (2014) White blood cell margination in microcirculation. Soft Matter 10:2961–2970
Freund JB (2013) The flow of red blood cells through a narrow spleen-like slit. Phys Fluids 25(11):110807
Fushimi K, Verkman AS (1991) Low viscosity in the aqueous domain of cell cytoplasm measured by picosecond polarization microfluorimetry. J Cell Biol 112:719–725
Groot RD, Warren PB (1997) Dissipative particle dynamics: bridging the gap between atomistic and mesoscopic simulation. J Chem Phys 107:4423–4435
Guo HL et al (2004) Mechanical properties of breast cancer cell membrane studied with optical tweezers. Chin Phys Lett 21:2543–2546
Helfrich W (1973) Elastic properties of lipid bilayers: theory and possible experiments. Z Naturforsch C C 28:693–703
Hoogerbrugge PJ, Koelman JMVA (1992) Simulating microscopic hydrodynamic phenomena with dissipative particle dynamics. Europhys Lett 19:155–160
Hou HW, Li QS, Lee GYH, Kumar AP, Ong CN, Lim CT (2009) Deformability study of breast cancer cells using microfluidics. Biomed Microdevices 11:557–564
Leong FY, Li QS, Lim CT, Chiam KH (2011) Modeling cell entry into a micro-channel. Biomech Model Mechanobiol 10:755–766
Li J, Dao M, Lim CT, Suresh S (2005) Spectrin-level modeling of the cytoskeleton and optical tweezers stretching of the erythrocyte. Biophys J 88:3707–3719
Li QS, Lee GYH, Ong CN, Lim CT (2008) AFM indentation study of breast cancer cells. Biochem Bioph Res Commun 374:609–613
Li X, Peng Z, Lei H, Dao M, Karniadakis GE (2014) Probing red blood cell mechanics, rheology and dynamics with a two-component multi-scale model. Philos Trans Ser A Math Phys Eng Sci 372. doi:10.1098/rsta.2013.0389
Lincoln B et al (2004) Deformability-based flow cytometry. Cytom Part A 59A:203–209
McDonald DM, Baluk P (2002) Significance of blood vessel leakiness in cancer. Cancer Res 62:5381–5385
Mierke CT (2011) Cancer cells regulate biomechanical properties of human microvascular endothelial cells. J Biol Chem 286:40025–40037
Mierke CT (2012) Endothelial cell’s biomechanical properties are regulated by invasive cancer cells. Mol Biosyst 8:1639–1649
Miles FL, Pruitt FL, van Golen KL, Cooper CR (2008) Stepping out of the flow: capillary extravasation in cancer metastasis. Clin Exp Metastasis 25:305–324
Omori T, Hosaka H, Imai Y, Yamaguchi T, Ishikawa T (2014) Numerical analysis of a red blood cell flowing through a thin micropore. Phys Rev E 89(1):013008
Pan DY, Nhan PT, Nam MD, Khoo BC (2013) Numerical investigations on the compressibility of a DPD fluid. J Comput Phys 242:196–210. doi:10.1016/j.jcp.2013.02.013
Park S, Ang RR, Duffy SP, Bazov J, Chi KN, Black PC, Ma HS (2014) Morphological differences between circulating tumor cells from prostate cancer patients and cultured prostate cancer cells. Plos One 9(1):e85264
Pivkin IV, Karniadakis GE (2008) Accurate coarse-grained modeling of red blood cells. Phys Rev Lett 101(11):118105
Quinn DJ, Pivkin I, Wong SY, Chiam KH, Dao M, Karniadakis GE, Suresh S (2011) Combined simulation and experimental study of large deformation of red blood cells in microfluidic systems. Ann Biomed Eng 39:1041–1050
Rejniak KA (2012) Investigating dynamical deformations of tumor cells in circulation: predictions from a theoretical model. Front Oncol 2:111. doi:10.3389/fonc.2012.00111
Reymond N, d’Agua BB, Ridley AJ (2013) Crossing the endothelial barrier during metastasis. Nat Rev Cancer 13:858–870
Schmidschonbein GW, Shih YY, Chien S (1980) Morphometry of human-leukocytes. Blood 56:866–875
Soares JS, Gao C, Alemu Y, Slepian M, Bluestein D (2013) Simulation of platelets suspension flowing through a stenosis model using a dissipative particle dynamics approach. Ann Biomed Eng 41:2318–2333
Stoletov K, Kato H, Zardouzian E, Kelber J, Yang J, Shattil S, Klemke R (2010) Visualizing extravasation dynamics of metastatic tumor cells. J Cell Sci 123:2332–2341
Strell C, Entschladen F (2008) Extravasation of leukocytes in comparison to tumor cells. Cell Commun Signal CCS 6:10. doi:10.1186/1478-811X-6-10
Sugihara-Seki M, Fu BMM (2005) Blood flow and permeability in microvessels. Fluid Dyn Res 37:82–132
Suresh S (2007) Biomechanics and biophysics of cancer cells. Acta Biomater 3:413–438
Voura EB, Sandig M, Siu CH (1998) Cell-cell interactions during transendothelial migration of tumor cells. Microsc Res Tech 43:265–275
Warren PB (2003) Vapor-liquid coexistence in many-body dissipative particle dynamics. Phys Rev E 68(6):066702
Weiss L, Orr FW, Honn KV (1988) Interactions of cancer-cells with the microvasculature during metastasis. Faseb J 2:12–21
Wirtz D, Konstantopoulos K, Searson PC (2011) The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer 11:512–522. doi:10.1038/nrc3080
Wu TH, Feng JJ (2013) Simulation of malaria-infected red blood cells in microfluidic channels: passage and blockage. Biomicrofluidics 7(4):044115
Yan WW, Cai B, Liu Y, Fu BM (2012) Effects of wall shear stress and its gradient on tumor cell adhesion in curved microvessels. Biomech Model Mech 11:641–653
Ye T, Phan-Thien N, Khoo BC, Lim CT (2014) Dissipative particle dynamics simulations of deformation and aggregation of healthy and diseased red blood cells in a tube flow. Phys Fluids 26(11):111902
Zhang ZF, Xu J, Hong B, Chen XL (2014) The effects of 3D channel geometry on CTC passing pressure-towards deformability-based cancer cell separation. Lab Chip 14:2576–2584
Zhu C, Bao G, Wang N (2000) Cell mechanics: mechanical response, cell adhesion, and molecular deformation. Annu Rev Biomed Eng 2:189–226
Acknowledgments
Supports given by HKRGC PolyU 5202/13E, PolyU G-YL41, NSFC 51276130, and NIH SC1 CA153325-01 are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiao, L.L., Liu, Y., Chen, S. et al. Numerical simulation of a single cell passing through a narrow slit. Biomech Model Mechanobiol 15, 1655–1667 (2016). https://doi.org/10.1007/s10237-016-0789-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-016-0789-y