Abstract
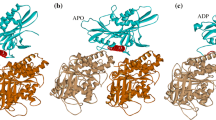
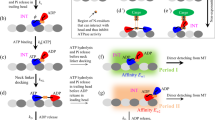
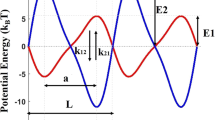
Kinesin is a motor protein that delivers cargo inside a cell. Kinesin has many different families, but they perform basically same function and have same motions. The walking motion of kinesin enables the cargo delivery inside the cell. Autoinhibition of kinesin is important because it explains how function of kinesin inside a cell is stopped. Former researches showed that tail binding is related to autoinhibition of kinesin. In this work, we performed normal mode analysis with elastic network model using different conformation of kinesin to determine the effect of tail binding by considering four models such as functional form, autoinhibited form, autoinhibited form without tail, and autoinhibited form with carbon structure. Our calculation of the thermal fluctuation and cross-correlation shows the change of tail-binding region in structural motion. Also strain energy of kinesin showed that elimination of tail binding effect leads the structure to have energetically similar behavior with the functional form.








Similar content being viewed by others
References
Asbury CL, Fehr AN, Block SM (2003) Kinesin moves by an asymmetric hand-over-hand mechanism. Science 302(5653):2130–2134
Atilgan AR, Durell SR, Jernigan RL, Demirel MC, Keskin O, Bahar I (2001) Anisotropy of fluctuation dynamics of proteins with an elastic network model. Biophys J 80(1):505–515
Bahar I, Atilgan AR, Erman B (1997) Direct evaluation of thermal fluctuations in proteins using a single-parameter harmonic potential. Fold Des 2(3):173–181
Bahar I, Rader AJ (2005) Coarse-grained normal mode analysis in structural biology. Curr Opin Struct Biol 15(5):586–592
Bahar I, Chennubhotla C, Tobi D (2007) Intrinsic dynamics of enzymes in the unbound state and relation to allosteric regulation. Curr Opin Struct Biol 17(6):633–640
Bahar I, Lezon TR, Yang L-W, Eyal E (2010) Global dynamics of proteins: bridging between structure and function. Annu Rev Biophys 39(1):23–42
Buehler MJ, Yung YC (2009) Deformation and failure of protein materials in physiologically extreme conditions and disease. Nat Mater 8(3):175–188
Carter NJ, Cross RA (2005) Mechanics of the kinesin step. Nature 435(7040):308–312
Clancy BE, Behnke-Parks WM, Andreasson JOL, Rosenfeld SS, Block SM (2011) A universal pathway for kinesin stepping. Nat Struct Mol Biol 18(9):1020–1027
Coy DL, Hancock WO, Wagenbach M, Howard J (1999) Kinesin’s tail domain is an inhibitory regulator of the motor domain. Nat Cell Biol 1(5):288–292
Czövek A, Szöllősi Gergely J, Derényi I (2011) Neck-linker docking coordinates the kinetics of kinesin’s heads. Biophys J 100(7):1729–1736
Dietrich KA, Sindelar CV, Brewer PD, Downing KH, Cremo CR, Rice SE (2008) The kinesin-1 motor protein is regulated by a direct interaction of its head and tail. Proc Natl Acad Sci 105(26):8938–8943
Guydosh NR, Block SM (2006) Backsteps induced by nucleotide analogs suggest the front head of kinesin is gated by strain. Proc Natl Acad Sci 103(21):8054–8059
Guydosh NR, Block SM (2009) Direct observation of the binding state of the kinesin head to the microtubule. Nature 461(7260):125–128
Haliloglu T, Bahar I, Erman B (1997) Gaussian dynamics of folded proteins. Phys Rev Lett 79(16):3090
Hirokawa N (1998) Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 279(5350):519–526
Hoeprich Gregory J, Thompson Andrew R, McVicker Derrick P, Hancock William O, Berger Christopher L (2014) Kinesin’s neck-linker determines its ability to navigate obstacles on the microtubule surface. Biophys J 106(8):1691–1700
Hyeon C, Onuchic JN (2007) Internal strain regulates the nucleotide binding site of the kinesin leading head. Proc Natl Acad Sci 104(7):2175–2180
Hyeon C, Onuchic JN (2011) A structural perspective on the dynamics of kinesin motors. Biophys J 101(11):2749–2759
Kaan HYK, Hackney DD, Kozielski F (2011) The structure of the kinesin-1 motor–tail complex reveals the mechanism of autoinhibition. Science 333(6044):883–885
Knowles TPJ, Buehler MJ (2011) Nanomechanics of functional and pathological amyloid materials. Nat Nanotechnol 6(8):469–479
Mather WH, Fox RF (2006) Kinesin’s biased stepping mechanism: amplification of neck linker zippering. Biophys J 91(7):2416–2426
Miyashita O, Onuchic JN, Wolynes PG (2003) Nonlinear elasticity, proteinquakes, and the energy landscapes of functional transitions in proteins. Proc Natl Acad Sci USA 100(22):12570–12575
Paparcone R, Buehler MJ (2011) Failure of A\({\upbeta }\)(1–40) amyloid fibrils under tensile loading. Biomaterials 32(13):3367–3374
Paparcone R, Keten S, Buehler MJ (2010) Atomistic simulation of nanomechanical properties of Alzheimer’s A\(\upbeta \)(1–40) amyloid fibrils under compressive and tensile loading. J Biomech 43(6):1196–1201
Scarabelli G, Grant BJ (2013) Mapping the structural and dynamical features of kinesin motor domains. PLoS Comput Biol 9(11):e1003329
Tirion MM (1996) Large amplitude elastic motions in proteins from a single-parameter, atomic analysis. Phys Rev Lett 77(9):1905
Togashi Y, Yanagida T, Mikhailov AS (2010) Nonlinearity of mechanochemical motions in motor proteins. PLoS Comput Biol 6(6):e1000814
Verhey KJ, Lizotte DL, Abramson T, Barenboim L, Schnapp BJ, Rapoport TA (1998) Light chain-dependent regulation of Kinesin’s interaction with microtubules. J Cell Biol 143(4):1053–1066
Verhey KJ, Hammond JW (2009) Traffic control: regulation of kinesin motors. Nat Rev Mol Cell Biol 10(11):765–777
Verhey KJ, Kaul N, Soppina V (2011) Kinesin assembly and movement in cells. Annu Rev Biophys 40(1):267–288
Xu CY, Tobi D, Bahar I (2003) Allosteric changes in protein structure computed by a simple mechanical model: hemoglobin T \(\leftrightarrow \) R2 transition. J Mol Biol 333(1):153–168
Xu Z, Paparcone R, Buehler MJ (2010) Alzheimer’s A\(\upbeta \)(1–40) amyloid fibrils feature size-dependent mechanical properties. Biophys J 98(10):2053–2062
Yang Z, Májek P, Bahar I (2009) Allosteric transitions of supramolecular systems explored by network models: application to chaperonin GroEL. PLoS Comput Biol 5(4):e1000360
Yoon G, Kwak J, Kim JI, Na S, Eom K (2011) Mechanical characterization of amyloid fibrils using coarse-grained normal mode analysis. Adv Funct Mater 21(18):3454–3463
Zheng W, Doniach S (2003) A comparative study of motor-protein motions by using a simple elastic-network model. Proc Natl Acad Sci USA 100(23):13253–13258
Zheng W, Brooks BR, Hummer G (2007) Protein conformational transitions explored by mixed elastic network models. Proteins Struct Funct Bioinform 69(1):43–57
Acknowledgments
S.N. gratefully acknowledges the financial support from Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (MSIP) (No. 2007-0056094) and (No. 2013-055175). H.J.C. is grateful to the financial support from Global Ph.D. Fellowship Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. 2014H1A2A1021042).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jae In Kim and Hyun Joon Chang have made equal contributions in this work.
Rights and permissions
About this article
Cite this article
Kim, J.I., Chang, H.J. & Na, S. Identification of tail binding effect of kinesin-1 using an elastic network model. Biomech Model Mechanobiol 14, 1107–1117 (2015). https://doi.org/10.1007/s10237-015-0657-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-015-0657-1