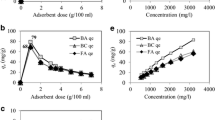
Abstract
Pyrite ash (PA), a waste produced during the roasting of pyrite ores to produce sulfuric acid, was studied as a potential adsorbent for removing arsenic (As) from groundwater. The collected pyrite ash waste samples contained >86 % iron (as Fe2O3). The results indicate that adsorption of As by PA was only slightly affected by initial pH at pH ≤ 9. Arsenate removal efficiency increased with the amount of adsorbent added over the range of 0.1–50 g/L. The As(V) removal increased with time, and 79 % removal was achieved within 1 h. Moreover, there was no significant change in As concentrations after 24 h. The adsorption process was best described by a second-order kinetic model. The adsorption of As(V) onto the PA was found to have followed the Langmuir isotherm. In batch studies, the maximum As(V) removal efficiency was 97 % at an adsorbent dose of 10 g/L, with an initial As(V) concentration of 300 µg/L. Thus, the PA was shown to be a suitable sorbent, reducing As from an initial level of 600 to <10 μg/L As(V), i.e., below the WHO limit for drinking water.
Zusammenfassung
Pyrit-Asche (PA), ein Abprodukt bei der Röstung pyritischer Erze für die Schwefelsäureherstellung, wurde als potenzielles Adsorptionsmittel für die Entfernung von Arsen (As) aus Grundwasser untersucht. Die Proben der PA enthielten >86% Fe2O3. Die Ergebnisse zeigen für pH≤9, dass die Adsorption von As an PA nur wenig vom anfänglichen pH beeinflusst wird. Die Entfernung von Arsenat stieg mit der eingesetzten Adsorptionsmittelmenge im Bereich 0,1-50 g/L. Die Entfernung von As(V) wuchs mit der Zeit und 79% wurden innerhalb einer Stunde erreicht. Danach gab es keine signifikante Änderung der As-Konzentration innerhalb von 24 Stunden. Die Adsorption konnte am besten mit einer Kinetik zweiter Ordnung beschrieben werden. Die Adsorption von As(V) an PA folgte der Langmuir-Isotherme. In Batch-Versuchen mit ursprünglich 300 µg/L As(V) wurden maximal 97% As(V) entfernt bei einem Einsatz von 10 g/L PA. Damit wurde gezeigt, dass PA ein geeignetes Adsorptionsmittel ist, das die As(V)-Konzentration von 600 µg/L auf <10µg/L senkt, also unter den WHO-Grenzwert für Trinkwasser.
Resumen
Las cenizas de pirita (PA), un residuo producido durante la tostación de minerales de pirita para producir ácido sulfúrico, fue estudiado como sorbente para remover (As) de agua subterránea. Las muestras de residuos de cenizas de pirita contenían >86% hierro (como Fe2O3). Los resultados indicaron que la adsorción de As por PA fue sólo ligeramente afectada por el pH inicial a pH ≤ 9. La eficiencia de la remoción de arseniato se incrementó con la cantidad de adsorbente agregado en el rango de 0,1-50 g/L. La remoción de As(V) se incrementó con el tiempo y una remoción de 79% se alcanzó en 1 h. Además, no hubo cambios significativos en las concentraciones de As luego de las 24 h. El proceso de adsorción se describe adecuadamente por un modelo cinético de segundo orden. La adsorción de As(V) sobre PA siguió una isoterma tipo Langmuir. En estudios en batch, la máxima eficiencia en la remoción de As(V) fue 97% con 10 g/L de adsorbente y una concentración inicial de As(V) de 300 µg/L. Estos resultados muestran que PA es un sorbente adecuado capaz de reducir la concentración de As desde un nivel inicial de 600 μg/L a <10 μg/L As(V), que es por debajo del límite WHO para agua de consumo humano.
抽象
黄铁矿灰渣(PA)是在焙烧黄铁矿石制硫酸的过程中产生的废物。研究了黄铁矿灰渣作为吸附剂去除地下水中砷(As)的潜的能力。黄铁矿灰渣样品的含铁量(以Fe2O3形式)大于86%。研究结果表明,在pH≤ 9的条件下,黄铁矿灰渣的砷(As)吸附作用仅受初始pH值影响。吸附剂含量超过0.1-50 g/L以后,砷酸盐(Arsenate)去除率随吸附剂含量增加而提高。五价砷As(V)的去除率随时间延长而提高,1个小时内就可达79%。在反应24小时之后,砷(As)浓度变化不再明显。吸附反应过程符合二级反应动力学模型。在五价砷As(V)初始浓度300µg/L和吸附剂含量10g/L的批次试验中,五价砷As(V)的最大去除率达97%;黄铁矿灰渣(PA)对五价砷As(V)的吸附反应遵循Langmuir isotherm吸附等温曲线。试验证明黄铁矿灰渣(PA)是一种理想的砷吸附剂,能够将砷的浓度由600 μg/L降到10μg/L,低于世卫组织饮用水砷浓度界限。






Similar content being viewed by others
References
Aksoy N, Simsek C, Gunduz O (2009) Groundwater contamination mechanisms in a geothermal field: a case study of Balcova, Turkey. J Contam Hydrol 103:13–28
Alp I, Deveci H, Yazıcı EY, Türk T, Sungun H (2009) Potential use of pyrite cinders as raw material in cement production: results of industrial scale trial operations. J Hazard Mater 166:144–149
Altundoğan HS, Altundoğan S, Tümen F, Bildik M (2000) Arsenic removal from aqueous solutions by adsorption on red mud. Waste Manag 20:761–767
Arienzo M, Adamo P, Chiaenzelli J, Bianco MR, Martino A (2002) Retention of arsenic on hydrous ferric oxides generated by electrochemical peroxidation. Chemosphere 48:1009–1018
Aydın AO, Gulensoy H, Akıcıoğlu A, Sakarya A (2003) Effect of boric acid and borax production of arsenic in colemanite. J Sci Technol Inst 5:51–58
Balsamo M, Di Natale F, Erto A, Lancia A, Montagnaro F, Santoro L (2010) Arsenate removal from synthetic wastewater by adsorption onto fly ash. Desalination 263:58–63
Başkan BM, Pala A, Türkman A (2010) Arsenate removal by coagulation using iron salts and organic polymers. Ekoloji 19:69–76
Borah D, Senapati K (2006) Adsorption of Cd(II) from aqueous solution onto pyrite. Fuel 85:1929–1934
Bulut E, Özacar M, Şengil İA (2008) Equilibrium and kinetic data and process design for adsorption of Congo Red onto bentonite. J Hazard Mater 154:613–622
Chuang CL, Fan M, Xu M, Brown RC, Sung S, Saha B, Huang CP (2005) Adsorption of arsenic(V) by activated carbon prepared from oat hulls. Chemosphere 61:478–483
Clara M, Magalhães F (2002) Arsenic: an environmental problem limited by solubility. Pure Appl Chem 74:1843–1850
Colak M, Gemici U, Tarcan G (2003) The effects of colemanite deposits on the arsenic concentration of soil and groundwater in Igdekoy-Emet, Kutahya, Turkey. Water Air Soil Poll 149:127–143
Cong L (2004) Sorption/desorption of Arsenic to Nanometer Scale Magnetite. PhD Thesis, Rice Univ, Houston, TX
Dada AO, Olalekan AP, Olatunya AM, Dada O (2012) Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ onto phosphoric acid modified rice husk. J Appl Chem 3:38–45
Doğan M, Doğan AU (2007) Arsenic mineralization, source, distribution, and abundance in the Kutahya region of the Western Anatolia, Turkey. Environ Geochem Health 29:119–129
Dubinin MM (1960) The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surface. Chem Rev 60:235–266
EPA (2000) Technologies and Costs for Removal of Arsenic from Drinking Water. Report 815-R-00-028, United States Environmental Protection Agency, Office of Water, Washington DC
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Gebreyowhannes YB (2009) Effect of Silica and pH on Arsenic Removal by Iron-oxide Coated Sand. MSc Thesis Unesco-Ihe Institute for Water Education, Delft, the Netherlands
Genc H, Tjell JC, McConchie D, Schuiling O (2003) Adsorption of arsenic from water using neutralized red mud. J Colloid Interface Sci 264:327–334
Gimenez J, Martınez M, Pablo D, Rovira M, Duro L (2007) Arsenic sorption onto natural hematite, magnetite, and goethite. J Hazard Mater 141:575–580
Guo H, Stüben D, Berner Z (2007) Removal of arsenic from aqueous solution by natural siderite and hematite. Appl Geochem 22:1039–1051
Ho YS, McKay G (1998) Kinetic model for lead(II) sorption on to peat. Adsorp Sci Technol 16:243–255
Hobson JP (1969) Physical adsorption isotherms extending from ultra-high vacuum to vapor pressure. J Phys Chem 73:2720–2727
Huang HH, Lu MC, Chen JN (2001) Cataltic decomposition of hydrogen peroxide and 2-chlophenol with iron oxides. Water Res 35:2291–2299
Islam M, Mishra PC, Pate R (2011) Fluoride adsorption from aqueous solution by a hybrid thorium phosphate Composite. Chem Eng J 166:978–985
Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, Rhodes CJ, Valko M (2011) Arsenic: toxicity, oxidative stress and human disease, review. J Appl Toxicol 31:95–107
Kroutil O, Chval Z, Skelton AA, Predota M (2015) Computer simulations of quartz (101)–water interface over a range of pH values. J Phys Chem 119:9274–9286
Kundu S, Gupta AK (2007) Adsorption characteristics of As (III) from aqueous solution on iron oxide coated cement (IOCC). J Hazard Mater 142:97–104
Lenoble V, Bouras O, Deluchat V, Serpaud B, Bollinger JC (2002) Arsenic adsorption onto pillared clays and iron oxides. J Colloid Interface Sci 255:52–58
Makris KC, Sarkar D, Datta R (2006) Evaluating a drinking-water waste by-product as a novel sorbent for arsenic. Chemosphere 64:730–741
Meng X, Korfiatis GP, Bang S, Bang KW (2002) Combined effects of anions on arsenic removal by iron hydroxides. Toxicol Lett 133:103–111
Mohan D, Pittman CU (2007) Arsenic removal from water/wastewater using adsorbents-a critical review. J Hazard Mater 142:1–53
Mondal P, Majumder CB, Mohanty B (2006) Laboratory based approaches for arsenic remediation from contaminated water: recent developments. J Hazard Mater 137:464–479
Mukherjee A, Sengupta MK, Amir Hossain MA, Ahamed S, Das B, Nayak B, Lodh D, Rahman MM, Chakraborti D (2006) Arsenic contamination in groundwater: a global perspective with emphasis on the Asian scenario. J Health Popul Nutr 24:142–163
Pecenyuk SI (1999) The use of the pH at the point of zero charge for characterizing the properties of oxide hydroxides. Russ Chem Bull 48:1017–1023
Raji C, Anirudhan TS (1998) Batch Cr(VI) removal by polyacrylamide-grafted sawdust: kinetics and thermodynamics. Water Res 32:3772–3780
Ravenscroft P, Brammer H, Richards K (2009) Arsenic pollution: a global synthesis. Wiley, Blackwell, London
Salim M, Munekage Y (2009) Removal of arsenic from aqueous solution using silica ceramic: adsorption kinetics and equilibrium studies. Int J Environ Res 3:13–22
Singh DB, Prasad G, Rupainwar DC (1996) Adsorption technique for the treatment of As(V)-rich effluents. Colloid Surf 111:49–56
Smedley PL, Nicolli HB, Macdonald DMJ, Barros AJ, Tullio JO (2002) Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina. Appl Geochem 17:259–284
Song S, Lopez-Valdivieso A, Hernandez-Campos DJ, Peng C, Monroy-Fernandez MG, Razo-Soto I (2006) Arsenic removal from high-arsenic water by enhanced coagulation with ferric ions and coarse calcite. Water Res 40:364–372
Tombacz E, Majzık A, Horvat ZS, Illes E (2006) Magnetite in aqueous medium: coating ıts surface and surface coated with it. Rom Rep Phys 58(3):281–286
Torrens KD (1999) Evaluating arsenic removal technologies. Pollut Eng 31:25–27
TS 266 (2005) Drinking water. Turkish Standards Institute, Ankara
Tugrul N, Derun EM, Piskin M (2007) Utilization of pyrite ash wastes by pelletization process. Powder Technol 176:72–76
Türk T, Alp I, Deveci H (2009) Adsorption of As(V) from water using Mg–Fe-based hydrotalcite (FeHT). J Hazard Mater 171:665–670
Türk T, Alp I, Deveci H (2010) Adsorption of As(V) from water using nanomagnetite. J Environ Eng 136:399–404
Tuutijärvi T, Repo E, Vahala R, Sillanpää M, Chen G (2012) Effect of competing anions on arsenate adsorption onto maghemite nanoparticles. Chin J Chem Eng 20:505–514
Vaughan RL, Reed BE (2005) Modeling As(V) removal by a iron oxide impregnated activated carbon using the surface complexation approach. Water Res 39:1005–1014
Veli S, Akyüz B (2007) Adsorption of copper and zinc from aqueous solutions by using natural clay. J Hazard Mater 149:226–233
WHO (1981) Arsenic, environmental health criteria 18 IPCS international program on chemical safety. Vammalankırjapaino, Vammala
WHO (1993) guidelines for drinking-water quality. Volume 1: recommendations, 2nd Edit, World Health Organization, Geneva, Switzerland
WHO (1996) Guidelines for drinking-water quality. Health criteria and other supporting information. Geneva, Switzerland, pp 940–949
Wickramasinghe SR, Han B, Zimbron J, Shen Z, Karim MN (2004) Arsenic removal by coagulation and filtration: comparison of groundwaters from the United States and Bangladesh. Desalination 169:231–244
Xu YH, Nakajima T, Ohki A (2002) Adsorption and removal of arsenic(V) from drinking water by aluminium loaded Shirazu-zeolite. J Hazard Mater 92:275–278
Zazouli MA, Balarak D, Mahdavi Y (2013) Application of Azolla Filiculoides biomass for 2-chlorophenol and 4-chrorophenol removal from aqueous solutions. Iran J Health Sci 1:43–55
Zhang W, Singh P, Paling E, Delides S (2004) Arsenic removal from contaminated water by natural iron ores. Miner Eng 17:517–524
Zhang S, Liu C, Luan Z, Peng X, Ren H, Wang J (2008) Arsenate removal from aqueous solutions using modified red mud. J Hazard Mater 152:486–492
Zhou YT, Nie HL, Branford-White C, He ZY, Zhu LM (2009) Removal of Cu2+ from aqueous solution by chitosan-coated magnetic nanoparticles modified with α-ketoglutaric acid. J Coll Interface Sci 330:29–37
Acknowledgments
The authors thank the Karadeniz Technical University Scientific Research Fund (Project Kod No: 2013ARGEBD-9040) for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Turk, T. Removal of Dissolved Arsenic by Pyrite Ash Waste. Mine Water Environ 36, 255–263 (2017). https://doi.org/10.1007/s10230-016-0406-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-016-0406-4