Abstract
This paper examines how asymmetric information in pharmaceutical licensing affects the safety standards of licensed drugs. Pharmaceutical companies often license potential drug molecules at different stages of drug development from other pharmaceutical or biotechnology companies and complete the remaining of research stages before submitting the new drug application(NDA) to the food and drug administration. The asymmetric information associated with the quality of licensed molecules might result in the molecules which are less likely to succeed to be licensed out, while those with greater potential of success being held internally for development. We identify the NDAs submitted between 1993 and 2004 where new molecular entities were acquired through licensing. Controlling for other drug area specific and applicant firm specific factors, we investigate whether drugs developed with licensed molecules face higher probability of safety based recall and ultimate withdrawal from the market than drugs developed internally. Results suggest the opposite of Akerlof’s (Q J Econ 84:488–500, 1970) lemons problem. Licensed molecules rather have less probability of facing safety based recalls and ultimate withdrawal from the market comparing to internally developed drug molecules. This suggests that biotechnology and small pharmaceutical firms specializing in pharmaceutical research are more efficient in developing good potential molecules because of their concentrated research. Biotechnology firms license out good potential molecules because it increases their market value and reputation. In addition, results suggest that both the number of previous approved drugs in the disease area, and also the applicant firms’ total number of previous approvals in all disease areas reduce the probability that an additional approved drug in the same drug area will potentially be harmful.
Similar content being viewed by others
Notes
The pharmaceutical and biotechnology licensing is rising at an increasing rate from 1990 and between 1996 and 2000 it had increased to an average of 616 a year [3].
Though it is true that the true magnitude of the safety problem is not revealed until the drug is on the market but the licensors can predict the potentiality and safety profile through drug research and while passing through the different phases of research. Hidden data is a problem of the pharmaceutical industry and licensors do not necessarily disclose all the information related to the drug to the licensee or to the public. One example is the drug Tamiflu and the safety problem associated with it. The safety issues were known to the NDA applicant but not to public. Please see detail here http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4254b_09_01_Tamiflu%20AE%20Review%202006%20Redacted_D060309_092.pdf.
We thank Recombinat Capital for providing the database.
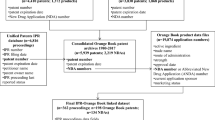
Please see http://people.hmdc.harvard.edu/~dcarpent/fdaproject.html for a detailed description.
As collected by Daniel Paul Carpenter’s FDA project.
We use multiple dummy variables for post-marketing safety problems because they are very different in nature and objective. The FDA codes these safety issues separately. Safety based withdrawals are complete withdrawal of drugs from the market and the BBW is a warning about the drug specified on drug label. After BBW physicians can still prescribe the drug after informing the patients about the warning. On the other hand the dosage-form discontinuation is a change in dosage or an approved version of NDA. Dosage-form discontinuation may or may not be related to same safety issues as BBW or recall. Therefore, separate dummy variables are required to test different post marketing safety problems. Please see [9] for a detailed discussion.
The stage of the licensing is available for each contract signed in the licensing database.
This result matches with [8] result though our data sets are different.
References
Akerlof, G.A.: The market for ‘lemons’: quality uncertainty and the market mechanism. Q. J. Econ. 84, 488–500 (1970) (The MIT Press)
Avorn, J.: Powerful Medicines: The Benefits, Risks, and Costs of Prescription Drugs. Knopf, New York (2004)
Arnold, K., Coia, A., Saywell, S., Smith, T., Minick, S., Löffler A.: Value drivers in licensing deals. Nat. Biotechnol. 20(11), 1085–1089 (2002)
Arora, A.: Licensing tacit knowledge: intellectual property rights and the market for know-how. Econ. Innov New Technol. 4, 41–59 (1995)
Arora, A., Alfonso, G., Fabio, P., Massimo, R.: The nature and the extent of the market for technology in biopharmaceuticals. In: Cesaroni, F., Gambardella, A., Garcia-Fontes, W. (eds.) R&D Innovation and Competitiveness in the European Chemical Industry, pp. 175–202. Springer, US (2004)
Banerjee, T., Nayak, A.: Effect of research and development outsourcing on new drug approvals in the pharmaceutical industry. J. Pharm. Health Serv. Res. 4, 51–56 (2013)
Beggs, A.W.: Licensing under asymmetric information. Int. J. Ind. Organ. 10, 170–191 (1992)
Begosh, A., Goldsmith, J., Haas, E., Lutter, R., Nardinelli, C., Vernon, J.: Black box warnings and drug safety: examining the determinants and timing of FDA warning labels NBER working paper, vol. 12803 (2006)
Carpenter, D., Zucker, E., Bowers, J., Grimmer, J., Moffitt, S., Nall, C.: Deadline effects in regulatory drug review: a methodological and empirical analysis, Robert Wood Johnson Foundation Scholars in Health Policy Research. Working Paper 35. George Washington University, Washington (2007)
Carpenter, D., Zucker, E., Avorn, J.: Drug-review deadlines and safety problems. N. Engl. J. Med. 358(13), 1354–1361 (2008)
Choi, J.P.: Technology transfer with moral hazard. Int. J. Ind. Organ. 19, 249–266 (2001)
Choi, J.P.: A dynamic analysis of Licensing: the “Boomerang” effect and grant-back clauses. Int. Econ. Rev. 43(3), 803–829 (2002)
Danzon, P.M., Nicholson, S., Pereira, N.S.: Productivity in Pharmaceutical-biotechnology R&D: the role of experience and alliances. J. Health Econ. 24(2), 317–339 (2005)
Gallini, N., Wright, B.: Technology transfer under asymmetric information. Rand J. Econ. 21, 147–160 (1990)
Kamien, M.I., Tauman, Y.: Patent licensing : the inside story. Manch Sch. 70, 7–15 (2002)
Katz, M., Shapiro, C.: Network externalities, competition and compatibility. Am. Econ. Rev. 75, 424–440 (1985)
Katz, M.L., Shapiro, C.: How to license intangible property. Q. J. Econ. 101, 567–590 (1986)
Lasser, K.E., Allen, P.D., Woolhandler, S.J., Himmelstein, D.U., Wolfe, S.M., Bor, D.H.: Timing of new Black box warnings and withdrawals for prescription medications. JAMA 287, 2215–20 (2002)
Macho-Stadler, I., Martinez-Giralt, X., Perez-Castrillo, D.J.: The Role of information in licensing contract design. Res. Policy 25, 43–57 (1996)
Nicholson, S., Danzon, P.M., McCullough, J.: Biotech-pharmaceutical alliances as a signal of asset and firm quality. J. Bus. 78(4), 1433–1464 (2005)
Powell, W.W., Koput, K.W., Smith-Doerr, L.: Interorganizational collaboration and the locus of innovation: networks of learning in biotechnology. Adm. Sci. Q. 41, 116–145 (1996)
Philipson, T., Berndtc, R.E., Gottschalkd, H.B.A., Sune, E.: Cost-benefit analysis of the FDA: the case of the prescription drug user fee acts. J. Public Econ. 92(5–6), 1306–1325 (2008)
Pisano, G.P.: R&D performance, collaborative arrangements and the arket for know-how: a test of the "Lemons" hypothesis in biotechnology. SSRN Electron J. doi:10.2139/ssrn.41980
Ray, W.A., Stein, C.M.: Reform of drug regulation–beyond an independent drug safety board. N. Engl. J. Med. 354(2), 194–201 (2006)
Rogers, M.J., Gupta, A., Maranas, C.D.: Real options based analysis of optimal pharmaceutical research and development portfolios. Ind. Eng. Chem. Res. 41, 6607–6620 (2002)
Schafer, D.P.: In-licensing as a business model. Nat. Biotechnol. 20, BE36–BE39 (2002)
Stuart, T.E., Hoang, H., Hybels, R.C.: Interorganizational endorsements and the performance of entrepreneurial ventures’. Adm. Sci. Q. 44(2), 315–349 (1999)
Susan, T.: Prescription Drug User Fee Act (PDUFA), Congressional Research Services Report RL33914, Congressional Research Services (2007)
Thaul, S., Domestic Social Policy Division.: The prescription drug user fee act (PDUFA): background and issues for PDUFA IV reauthorization. CRS report for congress, United States Congressional Research Service. http://www.amcp.org/WorkArea/DownloadAsset.aspx?id=11595 (2007)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Banerjee, T., Nayak, A. Why trash don’t pass? pharmaceutical licensing and safety performance of drugs. Eur J Health Econ 18, 59–71 (2017). https://doi.org/10.1007/s10198-015-0758-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-015-0758-x