Abstract
Purpose
To determine the cost effectiveness of lenalidomide plus dexamethasone (LEN/DEX) versus DEX alone in managing multiple myeloma (MM) patients who have failed one prior therapy.
Materials and Methods
An individual simulation model was designed to capture the costs and outcomes of LEN/DEX versus DEX therapy in relapsed refractory MM patients. MM009/010 efficacy data were adjusted for treatment cross-over and extrapolated to patient lifetime. Resource use for MM disease progression and adverse events were obtained from expert physicians and costed from the perspective of the National Health Service (England and UK) and included a patient access scheme for LEN. Utility values were obtained from published literature.
Results
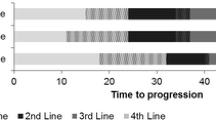
The simulation model estimated an incremental improvement in time to progression of 9.5 months, an additional 3.2 life-years, and 2.2 quality adjusted life years (QALY) for LEN/DEX compared to DEX alone. Including the costs of therapy with the patient access scheme, adverse events, and disease follow-up, the incremental cost effectiveness ratio was £30,153/QALY for LEN/DEX compared to DEX alone in MM patients who have failed one prior therapy.
Conclusion
LEN/DEX is a cost effective oncology therapy from the perspective of the NHS for MM patients with one prior treatment.


Similar content being viewed by others
References
Cancer Research UK: Multiple myeloma—UK incidence statistics. http://info.cancerresearchuk.org/cancerstats/types/multiplemyeloma/incidence/index.htm (2010). Accessed 19 July 2011
Scott, L.J., Lyseng-Williamson, K.A.: Lenalidomide: a review of its use in the treatment of relapsed or refractory multiple myeloma. Drugs 71, 625–649 (2011)
Schey, S., Higginson, I.: Cost-effectiveness of lenalidomide in multiple myeloma. Expert Rev. Pharmacoecon. Outcomes Res. 10, 229–238 (2010)
Drayson, M.T., Augustson, B.M., Begum, G., et al.: Survival from relapse and the influence of therapy (Abstract PO-665). Haematologica 92, 173 (2007)
Kumar, S.K., Rajkumar, S.V., Dispenzieri, A., et al.: Improved survival in multiple myeloma and the impact of novel therapies. Blood 111, 2516–2520 (2008)
Stadtmauer, E.A., Weber, D.M., Niesvizky, R., et al.: Lenalidomide in combination with dexamethasone at first relapse in comparison with its use as later salvage therapy in relapsed or refractory multiple myeloma. Eur. J. Haematol. 82, 426–432 (2009)
Dimopoulos, M.A., Palumbo, A., Attal, M., et al.: Optimizing the use of lenalidomide in relapsed or refractory multiple myeloma: consensus statement. Leukemia 25, 749–760 (2011)
Caro, J.J.: Pharmacoeconomic analyses using discrete event simulation. Pharmacoeconomics 23, 323–332 (2005)
Dimopoulos, M., Spencer, A., Attal, M., et al.: Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N. Engl. J. Med. 357, 2123–2132 (2007)
Weber, D., Knight, R., Chen, C. et al.: Prolonged overall survival with lenalidomide plus dexamethasone compared with dexamethasone alone in patients with relapsed or refractory multiple myeloma. Blood 110, 412 (2007)
National Institute for Health and Clinical Excellence: Guide to the methods of technology appraisal. http://www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf (June 2008). Accessed 19 July 2011
Durie, B.G., Harousseau, J.L., Miguel, J.S., et al.: International uniform response criteria for multiple myeloma. Leukemia 20, 1467–1473 (2006)
Ishak, K.J., Caro, J.J., Drayson, M.T., et al.: Adjusting for patient crossover in clinical trials using external data: a case study of lenalidomide for advanced multiple myeloma. Value Health 14, 672–678 (2011)
van Agthoven, M., Segeren, C.M., Buijt, I., et al.: A cost-utility analysis comparing intensive chemotherapy alone to intensive chemotherapy followed by myeloablative chemotherapy with autologous stem-cell rescue in newly diagnosed patients with stage II/III multiple myeloma; a prospective randomised phase III study. Eur. J. Cancer 40, 1159–1169 (2004)
Ossa, D.F., Briggs, A., McIntosh, E., Cowell, W., Littlewood, T., Sculpher, M.: Recombinant erythropoietin for chemotherapy-related anaemia: economic value and health-related quality-of-life assessment using direct utility elicitation and discrete choice experiment methods. Pharmacoeconomics 25, 223–237 (2007)
Lloyd, A., Nafees, B., Narewska, J., Dewilde, S., Watkins, J.: Health state utilities for metastatic breast cancer. Br. J. Cancer 95, 683–690 (2006)
Cykert, S., Joines, J.D., Kissling, G., Hansen, C.J.: Racial differences in patients’ perceptions of debilitated health states. J. Gen. Intern. Med. 14, 217–222 (1999)
Coffey, J.T., Brandle, M., Zhou, H., et al.: Valuing health-related quality of life in diabetes. Diabetes Care 25, 2238–2243 (2002)
Mathias, S.D., Putterman, C.G., Prebil, L.A., et al.: A health-related quality of life measure in patients with deep vein thrombosis: a validation study. Drug Inf. J. 33, 1173–1187 (1999)
Department of Health: The 2009 pharmaceutical price regulation scheme. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_091825 (December 11, 2008). Accessed 19 July 2011
Department of Health: NHS reference costs 2008–2009. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_111591 (2010). Accessed 19 July 2011
Richardson, P.G., Weller, E., Lonial, S., et al.: Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood 116, 679–686 (2010)
Richards, M.: Improving access to medicines for NHS patients: a report for the Secretary of State for Health by Professor Mike Richards. http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_089927 (November 4, 2008). Accessed 19 July 2011
Steinbach, R.: Inequalities in the distribution of health and health care and its access, including inequalities relating to social class, gender, culture and ethnicity, and their causes. Equality, equity and policy: inequalities in the distribution of health and health care and its access. http://www.healthknowledge.org.uk/public-health-textbook/medical-sociology-policy-economics/4c-equality-equity-policy/inequalities-distribution (2009). Accessed 19 July 2011
Department of Health: Improving outcomes: a strategy for cancer. http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_123394.pdf (January 2011). Accessed 29 Feb 2012
Acknowledgements
The authors wish to acknowledge statistical support from Jack Ishak, PhD, and production support from Fritz Hamme, BA, both of UBC. This work was supported by a grant from Celgene UK & Ireland. R.B. conducted the research and contributed to the manuscript. S. Stern constructed the economic model and contributed to the manuscript. S.D. advised on sensitivity analyses and the patient access plan, and contributed to the manuscript. S. Schey provided a clinical level review of research and the manuscript, and contributed to the manuscript’s discussion.
Conflict of interest
Ruth Brown and Sean Stern have received research funding from Celgene. Steve Schey has been compensated for his consultant role with Celgene. Sujith Dhanasiri works for Celgene and is compensated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brown, R.E., Stern, S., Dhanasiri, S. et al. Lenalidomide for multiple myeloma: cost-effectiveness in patients with one prior therapy in England and Wales. Eur J Health Econ 14, 507–514 (2013). https://doi.org/10.1007/s10198-012-0395-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-012-0395-6