Abstract
Background
This is the first case series to describe adjacent segment infection (ASI) after surgical treatment of spondylodiscitis (SD).
Materials and methods
Patients with SD, spondylitis who were surgically treated between 1994 and 2012 were included. Out of 1187 cases, 23 (1.94 %) returned to our institution (Zentralklinik Bad Berka) with ASI: 10 males, 13 females, with a mean age of 65.1 years and a mean follow-up of 69 months.
Results
ASI most commonly involved L3–4 (seven patients), T12–L1 (five) and L2–3 (four). The mean interval between operations of primary infection and ASI was 36.9 months. All cases needed surgical intervention, debridement, reconstruction and fusion with longer instrumentation, with culture and sensitivity-based postoperative antimicrobial therapy. At last follow-up, six patients (26.1 %) were mobilized in a wheelchair with a varying degree of paraplegia (three had pre-existing paralysis). Three patients died within 2 months after the ASI operation (13 %). Excellent outcomes were achieved in five patients, and good in eight.
Conclusions
Adjacent segment infection after surgical treatment of spondylodiscitis is a rare complication (1.94 %). It is associated with multimorbidity and shows a high mortality rate and a high neurological affection rate. Possible explanations are: haematomas of repeated micro-fractures around screw loosening, haematogenous spread, direct inoculation or a combination of these factors. ASI may also lead to proximal junctional kyphosis, as found in this series. We suggest early surgical intervention with anterior debridement, reconstruction and fusion with posterior instrumentation, followed by antimicrobial therapy for 12 weeks.
Level of evidence
Level IV retrospective uncontrolled case series.
Similar content being viewed by others
Introduction
Spondylodiscitis (SD) is a rare disease with incidence varying globally from one per 100,000 to one per 250,000/year [1, 2]. In many patients, clinical and imaging findings suggest the diagnosis before microbiological confirmation is obtained, and a causative organism remains unknown in up to 40 % of patients [2–4], causing greater difficulty for physicians in selecting the most appropriate antimicrobial treatment [5].
Although an elevation in C-reactive protein (CRP) and/or erythrocytic sedimentation rate (ESR) should not be taken as pathognomonic for an infection, both serve as screening and surveillance tests in the diagnosis and treatment of spinal infections [6]. The high sensitivity, specificity and accuracy of magnetic resonance imaging (MRI) make it the main imaging diagnostic tool in spinal infection [7]. A clinical diagnosis of spondylitis can be made in patients with positive blood cultures and compatible clinical history in combination with corresponding changes on laboratory and imaging studies. A definitive diagnosis of spondylitis can only be made on microscopic or bacteriological examination and culture of infected tissues [8].
Pyogenic infection in the postoperative period is a well documented complication of spinal surgery. In this case, the infection occurs mainly in the operated spinal segment. Adjacent segment infection (ASI) is a very uncommon complication [9].
To the best of the authors’ knowledge, no previously published study has described ASI after surgical treatment of SD. The current study aims to report and discuss this rare phenomenon.
Materials and methods
Study design
Single-centre, multi-surgeon, retrospective study of clinical and radiological outcome measures.
Patients
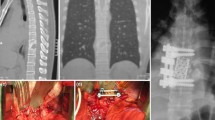
The medical database of our institution (Zentralklinik Bad Berka) was reviewed for patients with spinal infection who were surgically treated from 1994 to 2012. Patients with ASI were included. Patients with same level recurrent infection were excluded, as well as patients with ASI after surgery for spinal pathologies other than SD. Data were collected regarding demographics, presenting signs and symptoms, and predisposing and risk factors (Table 1). We also collected information regarding the level(s) of spinal involvement, perioperative inflammatory markers [white blood cell count (WBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP)], microbiological examination (blood cultures, intra-operative biopsy) and imaging modalities. Routinely, plain radiographs in anteroposterior and lateral views, and magnetic resonance imaging (MRI) of the whole spine routinely T1- and T2-weighted with and without contrast medium were performed. Additionally, computed tomography (CT) imaging was done in cases of marked bone destruction. This review also included the management of this phenomenon as regards antimicrobial treatment and surgical intervention, as well as surgical data, complications and outcomes (Figs. 1, 2, 3, 4).
The general condition of the patient was categorized according to the American Society of Anesthesiologists (ASA) score [10]. The ASA score is a subjective assessment of a patient’s overall health that is based on five classes:
-
1.
Patient is completely healthy and fit.
-
2.
Patient has mild systemic disease.
-
3.
Patient has severe systemic disease that is not incapacitating.
-
4.
Patient has incapacitating disease that is a constant threat to life.
-
5.
A moribund patient who is not expected to live for 24 h with or without surgery.
Data collection, assessment of the radiological findings and statistical analysis were performed by the first author (AES) and critically revised by the others. The adjacent segment lordosis angle was measured between the endplates above and below on the lateral views of postoperative radiographs and compared to those at the time of presentation with ASI (Table 2).
A diagnosis of spondylodiscitis was made on clinical, radiological and microbiological grounds, with patients fulfilling the following criteria:
-
1.
Clinical symptoms suggestive of spondylodiscitis (back pain unrelieved by rest; radiating pain ± neurological deficits ± fever) with laboratory abnormalities: WBC, ESR and CRP levels.
-
2.
Abnormal MRI (and other imaging modalities) features compatible with infection of the spine.
-
3.
Isolation of the causative microorganism or typical histological pattern from percutaneous disk or epidural abscess puncture or biopsy.
ASI was defined similarly, with infection of the adjacent segment (vertebra or intervertebral body) after surgical treatment of the primarily treated segment(s).
Treatment
All patients underwent operative treatment primarily and secondarily. The surgical approach was either posterior, anterior or combined anterior and posterior, with debridement, fusion and longer instrumentation. Autologous bone graft was harvested via a separate incision from the iliac crest.
Unless general health condition or intra-operative complications precluded it, all patients were mobilized with assistance on the first postoperative day. Postoperative treatment included a culture-based antimicrobial therapy, or a broad-spectrum antimicrobial therapy when no organism was isolated. This was given for a mean of 12 weeks and was stopped according to clinical, laboratory and radiological findings of recovery. After ASI surgery, antimicrobial therapy was continued for at least 12 weeks in all patients.
Follow-up
Preoperative, postoperative and last follow-up (FU) neurological findings were assessed according to Frankel’s classification: [11].
-
1.
‘Complete’ (A). Paralysis, both motor and sensory, below the level marked.
-
2.
‘Sensory only’ (B). Some sensation present below the level of the lesion but motor paralysis complete below that level.
-
3.
‘Motor Useless’ (C). Some motor power present below the lesion but of no practical use to the patient.
-
4.
‘Motor Useful’ (D). Useful motor power below the level of the lesion.
-
5.
‘Recovery’ (E). Free of neurological symptoms.
The final functional outcome was completed by questionnaires including Odom’s criteria [12] which categorized patients’ satisfaction into four grades: excellent, good, fair and poor.
-
Excellent: all preoperative symptoms relieved, abnormal findings unchanged or improved.
-
Good: minimum residual of preoperative symptoms not requiring medication or limiting activity, and abnormal findings unchanged or improved.
-
Fair: definite relief of some preoperative symptoms with others remaining unchanged or only slightly improved.
-
Poor: symptoms and signs unchanged from preoperative status or worse.
Statistical analysis
Descriptive statistics were used. Quantitative variables (e.g. age, laboratory values, operative data, interval between infections) were summarized by mean value and the standard deviation if appropriate. Qualitative demographic variables (e.g. gender and disease characteristics as well as potential prognostic factors) were summarized by counts and percentages. Analytical statistics were used to compare the preoperative and postoperative values as regards the laboratory findings. Because of the small number of cases, non-parametric tests were used, in this case the Wilcoxon signed-rank test. The same test was used to analyse the difference between the lordosis angle of the adjacent segment after primary surgery and at the time of presentation with ASI. To analyse the possible correlation between different variables and the outcomes according to Odom’s criteria, the Kruskal–Wallis test was used. Statistical significance was defined as p < 0.05. The statistical analysis was performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Demography
Between 1994 and 2012, 1187 patients were surgically treated in Zentralklinik Bad Berka because of SD. Out of these, 23 (10 males, 13 females) returned with ASI (1.94 %), with a mean age 65.1 ± 10.9 years. The primary infection was lumbar in 13 (56.5 %), thoracolumbar in four (17.4 %), thoracic in three (13 %), cervical in one (4.3 %) and combined thoracic and lumbar in two cases (8.7 %). Single-level infection was found in 16 patients (65.6 %), double-level in four (17.6 %) and three levels in three (13 %). Comorbidities were found in 19 patients (82.6 %); most commonly hypertension (HT) (12 patients, 52.2 %), diabetes mellitus (DM) (7, 30.4 %), osteoporosis (5, 21.7 %) and ischemic heart disease (IHD) (5, 21.7 %) (Table 1). The general condition of the patients before the primary surgery was ASA 1 in two patients (8.7 %), ASA 2 in six (26.1 %), ASA 3 in eight (34.8 %) and ASA 4 in seven (30.4 %). This distribution reflects the generally bad condition of these patients (Table 1).
Clinical presentation
At the time of primary infection, the main symptoms were back pain in 16 patients (69.6 %) and neurological deterioration in six (26.1 %). The average period of conservative treatment was 2.17 months before surgery (Table 2).
At the time of treatment of ASI, patients presented most commonly with recurrence of severe back pain (15 cases, 65.2 %). The mean interval between the operation of primary infection and the operation of ASI was 36.88 months.
Neurologically, one patient had Frankel grade B paraplegia (4.3 %), six patients had paraparesis grade C (26.1 %), one had grade D (4.3 %), and 15 patients (65.2 %) were neurologically free (grade E).
Laboratory findings
The mean preoperative laboratory values were WBC 9830 ± 4743/mm3, ESR 77.8 ± 36.7 mm/h and CRP 94.8 ± 77.2 mg/dL. The difference in relation to the immediate postoperative values was not statistically significant (p = 0.813, 0.465 and 0.594, respectively). At readmission with ASI, the mean values were WBC 10,496 ± 6697/mm3, ESR 79.8 ± 37 mm/h and CRP 94 ± 83 mg/dL. Postoperative values did not differ significantly after ASI operation (p = 0.859, 0.345 and 0.889, respectively).
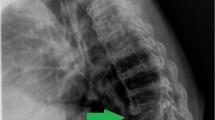
Diagnostic imaging
The most common primarily involved levels were L3–4 (seven, 30.4 %), L4–5 (seven, 30.4 %) and L2–3 (five, 21.7 %). ASI most commonly involved L3–4 (seven, 30.4 %), T12–L1 (five, 21.7 %) and L2–3 (four, 17.4 %). ASI involved cranial segment in ten patients (43.5 %), caudal segment in ten (43.5 %), floating segment in two (8.7 %) and adjacent segments cranially and caudally in one case (4.3 %), mono-segmental affection in 19 cases (82.6 %), bi-segmental in seven cases (30.4 %) and multi-segmental in one case (4.3 %).
Multifocal non-contiguous spinal infection was diagnosed in four patients (17.4 %); two cervical and two thoracic spinal infections coincided with lumbar infection. An epidural abscess was found in four patients (17.4 %) and psoas abscess in seven (30.4 %).
Primary surgery
The mean operative time was 217 ± 69.5 min with a mean blood loss of 1223 ± 710 ml. Mono- and bi-segmental spinal fusions were done in seven (30.4 %) and eight (34.8 %) patients, respectively. Three-, four- and five-segment fusions were performed in five patients (21.7 %), two (8.7 %) and one (4.3 %), respectively. Interbody fusion was done in 16 patients (69.6 %), while corpectomy was done in seven patients (30.4 %). Eight patients had bone graft only (34.8 %), and 15 had bone graft and cage (65.2 %). Minimally invasive techniques (e.g. video-assisted thoracoscopic surgery and percutaneous instrumentation) were used in six patients (26.1 %), while an open technique was used in 17 patients (73.9 %) (Table 3).
Postoperative treatment
A broad-spectrum antimicrobial was started on the same day after surgery (or continued), and was shifted according to culture and sensitivity tests. The most common causative organism identified (in primary SD) was Staphylococcus aureus, in five patients (21.7 %). In 8 patients, no organism could be isolated (34.8 %) (Table 3).
Surgery for adjacent segment infection
The mean operative time was 215 ± 106.8 min with a mean blood loss of 1241 ± 587.1 ml. Bi-segmental spinal fusions were done in six patients (26.1 %), while the majority of patients had long-segment fusions; five segments in five patients, and more than five segments in five patients (21.7 %). Interbody fusion was done in 21 patients (91.3 %), while corpectomy was done in only two patients (8.7 %). Ten patients had bone graft and cages (43.5 %), and 13 had bone graft only (56.5 %). Minimally invasive techniques were used in eight patients (34.8 %) and open technique in 15 patients (65.2 %) (Table 3). Of 11 patients with positive microbiological findings, eight (72.7 %) had a recurrence of the same micro-organism with multiple antimicrobial drug resistance and three (27.3 %) had a superadded infection with another organism.
Radiological results
Marked increase in adjacent segment kyphosis (>10°) occurred in six patients (26.1 %) and screw loosening was identified in 13 patients (56.5 %) at the time of presentation with ASI (Table 2).
Functional outcome
At the later presentation with ASI, two patients had deteriorated to grade C (8.7 %) and three had weakness grade D (13 %). The mean FU period was 69 ± 55.13 months after primary surgery. At last FU, six patients (26.1 %) were mobilized in a wheelchair with a varying degree of paraplegia (three had pre-existing paralysis). The others did not have neurological changes during the FU period (Table 1). Three patients died within 2 months after ASI operation because of sepsis and/or multi-organ failure (13 %). Subjectively, out of 18 surviving patients at the time of this study, an excellent outcome was achieved in five (27.8 %), good in eight (44.4 %), fair in four (22.2 %) and poor in one patient (5.6 %). This outcome was not significantly related to age, sex, region affected, neurological status or other variables, as statistically analysed in Table 4.
Discussion
To the best of the authors’ knowledge, no previously published study has described the prevalence of ASI after surgical fusion in SD. We conducted a PubMed/Medline search and review of the available literature up to December 2014. This phenomenon has been described only in three case reports: one lumbar in Germany [13] and two cervical in India [9, 14]. This study presents the first case series of ASI in the literature. The main limitations of this study are the variable treatment options, the wide variation in FU period and the retrospective design of the study with a low level of evidence.
Because of long conservative treatment of SD, no causative organism could be isolated in many patients previously confirmed to have this disease. The surgical approach with radical debridement, posterior stabilization and reconstruction of anterior column using expandable titanium cages is a widespread and accepted method [13]. Korovessis et al. have even shown that the use of titanium mesh cages may have a beneficial influence on eradication of infection and fusion [15]. In addition, Ruf et al. did not find any association between titanium cages and persistence or recurrence of infection [16]. The goals of surgical intervention are to preserve neurological function and to facilitate stable bony fusion without severe kyphosis. Procedures range from decompression, debridement and drainage to interbody fusion and grafting, and are decided on a case-by-case basis [2].
Based on the current study, we suggest early surgical intervention because of the higher incidence of multi-drug-resistant micro-organisms and before extension of bone destruction and expected deterioration of general condition and neurological functions of the patient. The usual surgical treatment consists of anterior debridement, reconstruction and fusion combined with open or percutaneous posterior instrumentation. This allows adequate eradication of the septic focus, resistance-adjusted antimicrobial therapy and early mobilization. Postoperative antimicrobial therapy should be immediately started (or continued) for a further 12 weeks, depending on the causative organism and culture and sensitivity examinations.
From the authors’ research into an explanation of this phenomenon, the following hypotheses are presented:
-
1.
Haematogenous infection route: prolonged preoperative and postoperative antimicrobial treatment should have minimized the risk of re-infection via this route [13]. In this study, eight patients (34.8 %) had positive blood cultures within the FU time.
-
2.
Direct infection of adjacent segment by intra-operatively contaminated screws. This was suspected by Lange et al., using cannulated screws [13]. No cannulated screws were used in our series, which opposes the hypothesis that bacteria are being shielded from antibiotic treatment within the cannulation of screws. We still suggest that direct contamination during surgery by faulty drilling or by cranially located screws may have a role in ASI, as also suggested by Kulkarni and Hee [14]. Seven patients had ASI with the same infecting organism as primary SD; four of these organisms had acquired more antimicrobial resistance.
-
3.
Screw loosening is a very important finding in 13 patients (56.5 %) in this series. We assume that slowly progressing loosening of screws causes repeated micro-fractures in pedicles and endplates. These micro-fractures lead to small haematomas in bone tissue in pedicles, endplates and most importantly in the endplate–disc attachment. Subsequent infection of these haematomas in previously infected and operated spinal region is likely to occur, especially in multi-morbid patients with poor general condition.
-
4.
Proximal junctional kyphosis (PJK) is a well-known complication of spinal instrumentation, especially with long-segment fusions [17, 18]. We assume that the adjacent segment infection is a direct cause of many cases of PJK. In the current study, six patients had marked increase in kyphosis prior to ASI. From the authors’ point of view, in cases of junctional kyphosis, ASI should be suspected and intra-operative biopsies should be sent to histopathology and microbiology for exclusion of low-grade or subclinical infection.
References
Acosta FL Jr, Chin CT, Quiñones H, Ames CP, Weinstein PR, Chou D (2004) Diagnosis and management of adult pyogenic osteomyelitis of the cervical spine. Neurosurg Focus 17:E2
Butler JS, Shelly MJ, Timlin M, Powderly WG, O’Byrne JM (2006) Non-tuberculous pyogenic spinal infection in adults: a 12-year experience from a tertiary referral centre. Spine 31:2695–2700
Carragee EJ (1997) Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am 79:874–880
Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ (2000) Haematogenous pyogenic spinal infections and their surgical management. Spine 25:1668–1679
D’Agostino C, Scorzolini L, Massetti AP, Carnevalini M, d’Ettorre G, Venditti M, Vullo V, Orsi GB (2010) A seven-year prospective study on spondylodiscitis: epidemiological and microbiological features. Infection 38:102–107
Schinkel C, Gottwald M, Andress HJ (2003) Surgical treatment of spondylodiscitis. Surg Inf 4(4):387–391
An HS, Seldomridge A, Spinal infections (2006) Diagnostic tests and imaging studies. Clin Orthop Relat Res 444:27–33
Cheung WY, Luk KD (2012) Pyogenic spondylitis. Int Orthop 36(2):397–404
Basu S, Sreeramalingam R (2012) Adjacent level spondylodiscitis after anterior cervical decompression and fusion. Indian J Orthop 46(3):360–363
Dripps RD (1963) New classification of physical status. Anesthesiol 24:111
Mannion AF, Elfering A, Staerkle R et al (2005) Outcome assessment in low back pain: how low can you go? Eur Spine J 14(10):1014–1026
Odom GL, Finney W, Woodhall B (1958) Cervical disk lesions. J Am Med Assoc 166:23–28
Lange T, Schulte TL, Bullmann V (2010) Two recurrences of adjacent spondylodiscitis after initial surgical intervention with posterior stabilization, debridement, and reconstruction of the anterior column in a patient with spondylodiscitis: a case report. Spine 35(16):E804–E810
Kulkarni AG, Hee HT (2006) Adjacent level discitis after anterior cervical discectomy and fusion (ACDF): a case report. Eur Spine J 15(5):559–563
Korovessis P, Repantis T, Iliopoulos P et al (2008) Beneficial influence of titanium mesh cage on infection healing and spinal reconstruction in hematogenous septic spondylitis: a retrospective analysis of surgical outcome of twenty-five consecutive cases and review of literature. Spine 33:E759–E767
Ruf M, Stoltze D, Merk HR, Ames M, Harms J (2007) Treatment of vertebral osteomyelitis by radical debridement and stabilization using titanium mesh cages. Spine 32(9):E275–E280
Ahn DK, Park HS, Choi DJ, Kim KS, Yang SJ (2010) Survival and prognostic analysis of adjacent segments after spinal fusion. Clin Orthop Surg 2(3):140–147
Kim HJ, Lenke LG, Shaffrey CI, Van Alstyne EM, Skelly AC (2012) Proximal junctional kyphosis as a distinct form of adjacent segment pathology after spinal deformity surgery: a systematic review. Spine 37(22 Suppl):S144–S164
Acknowledgments
Special thanks go to the team of the hospital library in Zentralklinik Bad Berka, Mrs. Zuellinger and Mrs. Stahl, for their invaluable efforts in collection of related scientific articles.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they do not have any potential conflict of interest.
Ethical standards
This study conforms to the 1964 Helsinki declaration and its later amendments. It was approved by the responsible Ethics Committee and because of its retrospective design an informed consent was not required.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Siam, A.E., El Saghir, H. & Boehm, H. Adjacent segment infection after surgical treatment of spondylodiscitis. J Orthopaed Traumatol 17, 41–51 (2016). https://doi.org/10.1007/s10195-015-0380-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10195-015-0380-9