Abstract
Objective
We evaluated patient drug adherence to and efficacy and safety of adalimumab (ADA) based on data collected from approximately 200 patients to retrospectively examine the best use of ADA in Japanese patients with longstanding rheumatoid arthritis (RA) managed in daily practice.
Methods
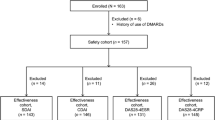
For explorative comparisons, patients were stratified by prior use or no use of biologics (Bio-naïve vs. Bio-switch) and concomitant use (+) or no use (−) of methotrexate (MTX) into four subgroups. The primary efficacy endpoint was extent of improvement in the Disease Activity Score in 28 joints using erythrocyte sedimentation rate (DAS28-ESR) from baseline to 24 weeks assessed as European League Against Rheumatism (EULAR) good response. Secondary endpoints included ADA treatment continuation as represented by Kaplan–Meier survival curves and percentages of patients achieving remission as defined by DAS28-ESR <2.6.
Results
Overall, mean DAS28-ESR significantly decreased from 5.6 ± 1.2 at baseline to 4.1 ± 1.7 at week 24 (p < 0.0001), and >30 % of patients achieved EULAR good response. Subgroup analyses indicated that patients in the Bio-naïve and MTX (+) subgroup showed the highest EULAR good response rate of 37.3 % at week 24. The three most commonly reported adverse events (AEs) were skin allergies such as injection-site reactions, infections, and respiratory disorders such as interstitial lung lesions and organizing pneumonia.
Conclusion
In conclusion, ADA therapy resulted in significant clinical response in established Japanese patients with RA treated in daily practice. It also demonstrated generally good safety and tolerability. It was suggested that the best use of ADA may be in biologically naïve patients with concomitant administration of MTX.



Similar content being viewed by others
References
Hochberg MC, Lebwohl MG, Plevy SE, Hobbs KF, Yocum DE. The benefit/risk profile of TNF-blocking agents: findings of a consensus panel. Semin Arthritis Rheum. 2005;34:819–36.
Vultaggio A, Matucci A, Nencini F, Pratesi S, Parronchi P, Rossi O, et al. Anti-infliximab IgE and non-IgE antibodies and induction of infusion-related severe anaphylactic reactions. Allergy. 2010;65:657–61.
Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti-tumor necrosis factor α monoclonal antibody, for treating rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45.
Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, et al. Radiographic, clinical and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50:1400–11.
Breedveld FC, Weisman MH, Kavanaugh AF, Cohen SB, Pavelka K, van Vollenhoven R, et al. A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54:26–37.
Weinblatt ME, Keystone EC, Furst DE, Kavanaugh AF, Chartash EK, Segurado OG. Long term efficacy and safety of adalimumab plus methotrexate in patients with rheumatoid arthritis: ARMADA 4 year extended study. Ann Rheum Dis. 2006;65:753–9.
Bejarano V, Quinn M, Conaghan PG, Reece R, Keenan A-N, Walker D, et al. Effect of the early use of the anti-tumor necrosis factor adalimumab on the prevention of job loss in patients with early rheumatoid arthritis. Arthritis Rheum. 2008;59:1467–74.
Van Vollenhoven RF, Cifaldi MA, Ray S, Chen N, Weisman MH. Improvement in work place and household productivity for patients with early rheumatoid arthritis treated with adalimumab plus methotrexate: work outcomes and their correlations with clinical and radiographic measures from a randomized controlled trial comparison study. Arthritis Care Res. 2010;62:226–34.
Kojima T, Kaneko A, Hirano Y, Ishikawa H, Miyake H, Oguchi T, et al. Study protocol of a multicenter registry of patients with rheumatoid arthritis starting biologic therapy in Japan: Tsurumai Biologics Communication Registry (TBCR) Study. Mod Rheumatol. 2011;. doi:10.1007/s10165-011-0518-4.
HUMIRA (adalimumab) (prescribing information) (in Japanese). Pharmaceuticals and medical devices agency web site. http://www.info.pmda.go.jp/go/pack/3999426G1024_1_12/.
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24.
Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–84.
Van Gestel AM, Haagsma CJ, van Riel PLCM. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum. 1998;41:1845–50.
Van Gestel AM, Anderson JJ, van Riel PL, Boers M, Haagsma CJ, Rich B, et al. ACR and EULAR improvement criteria have comparable validity in rheumatoid arthritis trials. American College of Rheumatology European League of Associations for Rheumatology. J Rheumatol. 1999;26:705–11.
Shinomiya F, Mima N, Nanba K, Tani K, Nakano S, Egawa H, et al. Life expectancies of Japanese patients with rheumatoid arthritis: a review of deaths over a 20-year period. Mod Rheumatol. 2008;18:165–9.
Van der Heijde D, Breedveld FC, Kavanaugh A, Keystone EC, Landewe R, Patra K, et al. Disease activity, physical function, and radiographic progression after long-term therapy with adalimumab plus methotrexate: 5-year results of PREMIER. J Rheumatol. 2010;37:2237–46.
Keystone EC, Kavanaugh A, Weinblatt ME, Patra K, Pangan AL. Clinical consequences of delayed addition of adalimumab to methotrexate therapy over 5 years in patients with rheumatoid arthritis. 2011. doi:10.3899/jrheum.100752.
Miyasaka N, the CHANGE study Investigators. Clinical investigation in highly disease-affected rheumatoid arthritis patients in Japan with adalimumab applying standard and general evaluation: the CHANGE study. Mod Rheumatol. 2008;18:252–62.
Bartelds GM, Wijbrandts CA, Nurmohamed MT, Stapel S, Lems WF, Aarden L, et al. Clinical response to adalimumab: relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritis. Ann Rheum Dis. 2007;66:921–6.
Radstake TRDJ, Svenson M, Eijsbouts AM, van den Hoogen FHJ, Enevold C, van Riel PLCM, et al. Formation of antibodies against infliximab and adalimumab strongly correlated with functional drug levels and clinical response in rheumatoid arthritis. Ann Rheum Dis. 2009;68:1739–45.
Kaneko A. Case reports of biological products: D. Adalimumab. In: Abe C, Kondo M, Matsubara T, Yamasaki K. Nihon Igakukan, editors. The manual of rheumatoid arthritis therapy with biologics. 2010; 69–77.
Kievit W, Adang EM, Fransen J, Kuper HH, van de Laar MAFJ, Jansen TL, et al. The effectiveness and medication costs of three anti-tumor necrosis factor agents in treating rheumatoid arthritis from prospective clinical practice data. Ann Rheum Dis. 2008;67:1229–34.
Hetland ML, Christensen IJ, Tarp U, Dreyer L, Hansen A, Hansen IT, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010;62:22–32.
Du Pan SM, Dehler S, Ciurea A, Ziswiler H-R, Gabay C, Finckh A. Comparison of drug retention rates and causes of drug discontinuation between anti-tumor necrosis factor agents in rheumatoid arthritis. Arthritis Rheum. 2009;61:560–8.
Gomez-Reino JJ, Garmona L. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther. 2006;8:R29–35.
Smolen JS, Landewé R, Breedveld FC, Dougados M, Emery P, Gaujoux-Viala C, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69:964–75.
Humira® (adalimumab) (prescribing information). Abbott Laboratories. North Chicago; 2010.
Paltiel M, Gober LM, Deng A, Mikdashi J, Alexeeva I, Saini SS, et al. Immediate type I hypersensitivity response implicated in worsening injection site reactions to adalimumab. Arch Dermatol. 2008;144:1190–4.
Benucci M, Manfredi M, Demoly P, Campi P. Injection site reactions to TNF-α blocking agents with positive skin tests. Allergy. 2008;63:138–9.
Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370:1861–74.
Conflict of interest
N. Ishiguro, T. Kojima, Y. Hirano, and A. Kaneko have received speaking fees (<US$5,000) from Abbott Japan Co. Ltd.; Eisai Co. Ltd.; Mitsubishi Tanabe Pharma Corporation; Pfizer Co. Ltd; Chugai Pharmaceutical Co. Ltd.; and Bristol-Myers Squibb Co. Ltd. The other authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kaneko, A., Hirano, Y., Fujibayashi, T. et al. Twenty-four-week clinical results of adalimumab therapy in Japanese patients with rheumatoid arthritis: retrospective analysis for the best use of adalimumab in daily practice. Mod Rheumatol 23, 466–477 (2013). https://doi.org/10.1007/s10165-012-0705-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10165-012-0705-y